An exothermic reaction releases energy to its surroundings.
![]() Watch the following video showing four different exothermic reactions.
Watch the following video showing four different exothermic reactions.
Source: 4 ways to make fire without matches by using chemistry, nurdrage, YouTube
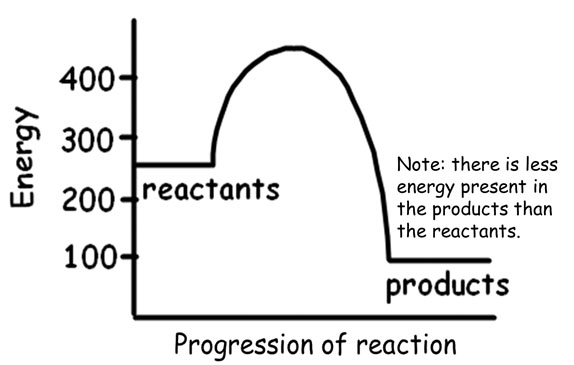
When a chemical bond is formed, energy is released making it an exothermic reaction. The heat released is also called enthalpy (heat content).
In an exothermic reaction, energy can be represented as a product.
X + Y → Z + 150 kJ
It can also be represented as having negative enthalpy.
|
X + Y → Z + 150 kJ
|
ΔH = -150kJ
|
![]() Sort the following items as endothermic or exothermic.
Sort the following items as endothermic or exothermic.
|
Exothermic Processes |
Endothermic Processes |
|
|
|
Exothermic Reactions |
Endothermic Reactions |
|
|