Carbon Family
Nitrogen Family
Oxygen Family
Halogen Family
Nobel Gases
Hydrogen
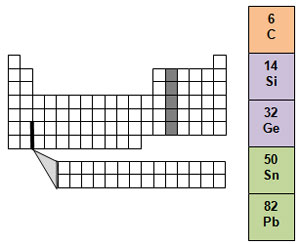
Group 14 is known as the carbon family. Only one of the elements is a nonmetal, and that element is carbon. Two of the elements in the group, silicon and germanium, are metalloids, while tin and lead are metals. Carbon is important because it is found in all living things.
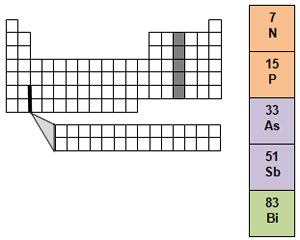
Group 15 is called the nitrogen family. It contains two nonmetals, nitrogen and phosphorus. Air is almost 80 percent nitrogen gas. Nitrogen does not easily combine with other elements. Nitrogen gas as it occurs in the air is not usable by living things. Only certain kinds of bacteria are able to combine nitrogen in the air with other elements. This process is called nitrogen fixation. Phosphorus is the other nonmetal in the nitrogen family. Phosphorus is always found in compounds.
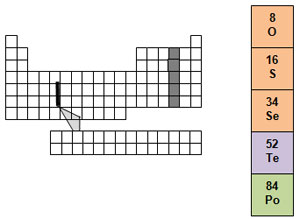
The oxygen family is found in group 16. It contains three nonmetals, oxygen, sulfur, and selenium. We breathe oxygen. You could not live without it. Oxygen is very reactive and can combine with almost every other element. It is also the most abundant element in Earth's crust and the second most abundant element in the atmosphere.
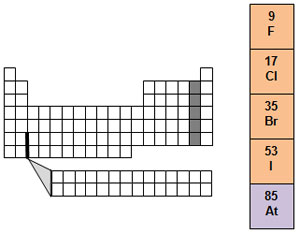
Group 17 is called the halogen family. It is made up of fluorine, chlorine, bromine, iodine, and the metalloid astatine. The word halogen means salt-forming. Halogens react with certain metals to form a class of compounds that include table salt. All halogens are very reactive. Most of them are dangerous to humans, but when combined with other elements, they are very useful.
Group 18 is the only group that is made up of all nonmetals. Group 18 is known as the noble gases. All the noble gases exist in Earth's atmosphere, but only in small amounts. Nobel gases are very stable and do not react with other elements.
Hydrogen is the simplest element. Because it only has one proton and one electron, it has very different chemical properties than all the other elements, so it really cannot be grouped in any other family.





