Alkali Metals
Alkali Earth Metals
Transition Metals
Lanthanides and Actinides
Metals in Mixed Groups
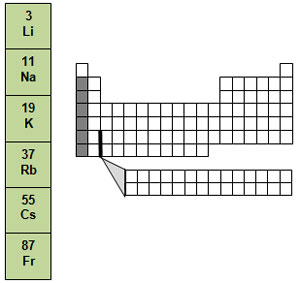
The metals in group 1, from lithium to francium, are called the alkali metals. They are the most reactive elements. In fact, they are never found uncombined in nature. They are always found as part of compounds. Alkali metals are very soft and shiny.
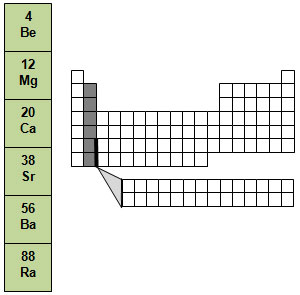
The metals found in group 2 are referred to as the alkaline earth metals. They are not as reactive as the metals in group 1, but they are still more reactive than most metals. They are also never found uncombined in nature. Each element in group 2 is fairly hard, bright white, and a good conductor of electricity.
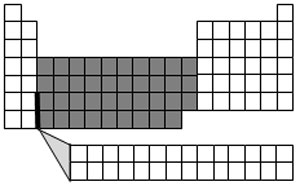
The elements found in groups 3 through 12 are called the transition metals. The transition metals form a bridge between the very reactive metals found in groups 1 and 2 and the less reactive metals, as well as other elements on the table. The transition metals are all very similar to each other. The transition metals include the metals you are probably most familiar with such as copper, nickel, silver, and gold. Most transition metals are hard and shiny and are good conductors of electricity. Transition metals react very slowly, if at all, with air and water.
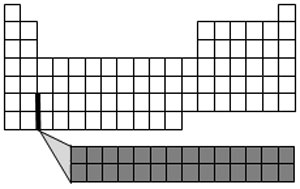
The bottom two rows of the periodic table are called the lanthanides (the top row) and actinides (the bottom row). These elements fit in periods 6 and 7 within the transition metals. These are also known as the rare earth metals. Most rare earth metals are silver, silvery-white, or gray and have a high luster. They are good conductors of electricity.
Groups 13 through 16 include metals, nonmetals, and metalloids. The metals that are found in these groups are not nearly as reactive as the other metals.




