What is the density of a liquid with a volume of 40mL and a mass of 100g?
A. .4 g/cm3
Incorrect. You divided volume by mass. The formula for density is mass divided by volume.
B. 2.5 g/cm3
Correct!
C. 4000 g/cm3
Incorrect. You multiplied the mass and the volume. The formula for density is mass divided by volume.
D. 60 g/cm3
Incorrect. You subtracted the volume from the mass. The formula for density is mass divided by volume.
A sample of an unknown metal has a mass of 528.64 grams and a volume of 59mL. Using the following table, identify the metal.
|
Metals and Their Densities |
|
|
Metal |
Density (g/cm3) |
|
Aluminum |
2.70 |
|
Zinc |
7.13 |
|
Iron |
7.87 |
|
Copper |
8.96 |
|
Silver |
10.49 |
|
Lead |
11.36 |
|
Mercury |
13.55 |
|
Gold |
19.32 |
A. iron
Incorrect. The formula for density is mass divided by volume. Try again.
B. gold
Incorrect. The formula for density is mass divided by volume. Try again.
C. Copper
Correct!
D. lead
Incorrect. The formula for density is mass divided by volume. Try again.
The following table shows four different sample materials. Which one of these would float on water? The density of water is 1 g/cm3.
|
Sample |
Mass (g) |
Volume (mL) |
|
A |
80 |
53 |
|
B |
35 |
10 |
|
C |
48 |
30 |
|
D |
52 |
75 |
A. sample A
Incorrect. The density of sample A is 1.5 g/cm3 which is more than 1 g/cm3, so
the sample would sink.
B. sample B
Incorrect. The density of sample B is 3.5 g/cm3 which is more than 1 g/cm3, so the sample would sink.
C. sample C
Incorrect. The density of sample C is 1.6 g/cm3 which is more than 1 g/cm3, so
the sample would sink.
D. sample D
Correct! The density of sample D is .69 g/cm3which
is less than 1 g/cm3 so this sample would float.
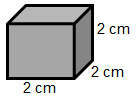
A student was given a piece of unknown metal pictured below. She determined the mass to be 90.88 grams. Identify the metal.
|
Metals and Their Densities |
|
|
Metal |
Density (g/cm3) |
|
Aluminum |
2.70 |
|
Zinc |
7.13 |
|
Iron |
7.87 |
|
Copper |
8.96 |
|
Silver |
10.49 |
|
Lead |
11.36 |
|
Mercury |
13.55 |
|
Gold |
19.32 |

A. lead
Correct! The volume of the sample is 8 g/cm3. The density would be 90.88 grams
divided by 8 cm3 which is 11.36 g/cm3.
B. zinc
Incorrect. The volume of the sample is 8 cm3. The density would be 90.88 grams
divided by 8 cm3.
C. iron
Incorrect. The volume of the sample is 8 cm3. The density would be 90.88 grams
divided by 8 cm3.
D. aluminum
Incorrect. The volume of the sample is 8 cm3. The density would be 90.88 grams
divided by 8 cm3.
A student was given the following information about an unknown element and instructed to identify the element. What is the element?
|
Initial volume of water |
25 mL |
|
Volume of water and sample |
57 mL |
|
Mass |
49.28 g |
|
Elements and Their Densities |
|
|
Elements |
Density (g/cm3) |
|
Carbon |
2.27 |
|
Sulfur |
2.07 |
|
Calcium |
1.54 |
|
Silicon |
2.33 |
|
Manganese |
7.44 |
|
Cobalt |
8.86 |
A. carbon
Incorrect. The volume would be 32 mL. To find the density, you would divided 49.28 by 32.
B. calcium
Correct! The volume would be 32 mL. To find the
density you would divided 49.28 by 32. The density would be 1.54 g/cm3.
C. silicon
Incorrect. The volume would be 32 mL. To find the density, you would divided 49.28 by 32.
D. cobalt
Incorrect. The volume would be 32 mL. To find the density, you would divided 49.28 by 32.