Which of the following statements best describes the subatomic particle identified as X on the diagram below?
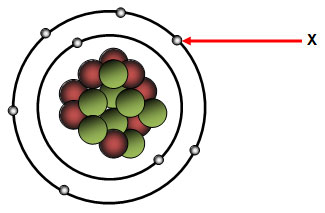
A. It has little or no mass and a neutral charge.
Incorrect. The subatomic particle is outside the nucleus there for it is an electron. Try again.
B. It has mass and a neutral charge.
Incorrect. The subatomic particle is outside the nucleus there for it is an electron. Try again.
C. It has mass and a negative charge.
Incorrect. The subatomic particle is outside the nucleus there for it is an electron. Try again.
D. It has little or no mass and a negative charge.
Correct! The subatomic particle is outside the nucleus there for it is an electron and electrons have little or no mass and a negative charge.
Which of the following describes a proton?
A. II, IV and V
Incorrect. Protons do have a mass, they are not found in the cloud, and they are not neutral.
B. I, III, IV
Incorrect. Protons do have a mass, they are not found in the cloud, and they are not neutral.
C. I and III
Correct! Protons have a mass and they have a positive charge.
D. II and IV
Incorrect. Protons do have a mass, they are not found in the cloud, and they are not neutral.
Use the atom drawing to identify the element. Click here to access the periodic table.
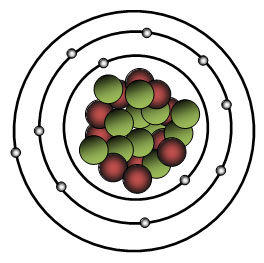
A. Neon
Incorrect. Count the number of electrons. The number of electrons is equal to the number of protons. The number of protons will tell us the atomic number.
B. Sodium
Correct! There are 11 electrons so there must be 11 protons and the atomic number must also be 11. The element with the atomic number of 11 is sodium.
C. Magnesium
Incorrect. Count the number of electrons. The number of electrons is equal to the number of protons. The number of protons will tell us the atomic number.
D. Oxygen
Incorrect. Count the number of electrons. The number of electrons is equal to the number of protons. The number of protons will tell us the atomic number.
According to the table below what is the atomic mass?
|
Subatomic Particle
|
#
|
|
Protons
|
19
|
|
Neutrons
|
10
|
|
Electrons
|
10
|
A. 19
Incorrect. The atomic mass is the number of protons and the number of neutrons.
B. 29
Correct! The atomic mass is the number of protons and the number of neutrons.
C. 9
Incorrect. The atomic mass is the number of protons and the number of neutrons.
D. 38
Incorrect. The atomic mass is the number of protons and the number of neutrons.