Many people mistakenly think that thermal energy, temperature and heat are the same thing. These three concepts are related, but they are in fact, three separate concepts.
You have learned that all matter is made up of particles (atoms or molecules). These particles move or vibrate constantly. Particles have both potential and kinetic energy due to their arrangement and motion.
The total energy of motion in the particles of a substance is called thermal energy. The thermal energy of an object depends on three factors:
- the arrangement of the object’s particles,
- the temperature of the object,
- and the number of particles in the object.
Arrangement of Particles
The image below shows the particles in a solid, liquid, and a gas.
Solid |
Liquid |
Gas |
|
|
|
In a solid, the particles are packed very closely together, and they are vibrating in place. |
In a liquid, the particles are not as closely packed and are moving faster than they are in solid form. |
In a gas, the particles are spread out even further and moving much faster than they are in the liquid form. |
Notice that in each picture the number of particles is the same. There are six particles in each picture. If we measured the thermal energy of each of these substances, which one would have the most thermal energy?
Temperature of the Object
Temperature is the average amount of energy of motion of each particle of a substance. The faster the molecules are moving around and/or vibrating, the higher the temperature. The higher the temperature of a substance, the more thermal energy the substance has.
The two cups of coffee pictured below contain the same number of particles. The temperature of the coffee in the cup on the left is 40oC while the temperature of the coffee in the cup on the right is 90°C. Which cup of coffee has more thermal energy?
The coffee in the cup on the right would have more thermal energy because the particles are moving at a much faster rate than the coffee in the cup on the left.
Temperature is measured using a thermometer. Thermometers work because liquids usually expand when heated and contract when they are cooled.
Number of Particles
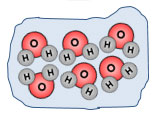
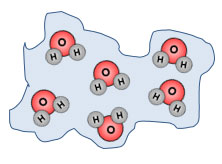
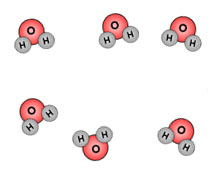
Look at these two images. Which one do you think has more thermal energy?
The iceberg has more thermal energy because there are more particles. Remember thermal energy is the total energy of motion in the particles of a substance. The iceberg has many more particles than the candle; therefore the iceberg has more thermal energy.
Let’s apply the concepts you have learned.
The image on the left shows a bucket of water at 50°C. The image on the right shows a cup of water at 100°C.
In which container would the water molecules be moving faster?

Source: Bucket of Water, Clker
In which container would the water have more thermal energy?
Thermal energy that is transferred from matter at a higher temperature to matter at a lower temperature is called heat. Thermal energy is transferred in three ways: conduction; convection; radiation.