Source: Iced Tea, Renee Comet, Wikimedia Commons

Source: Iced Tea, Renee Comet, Wikimedia Commons
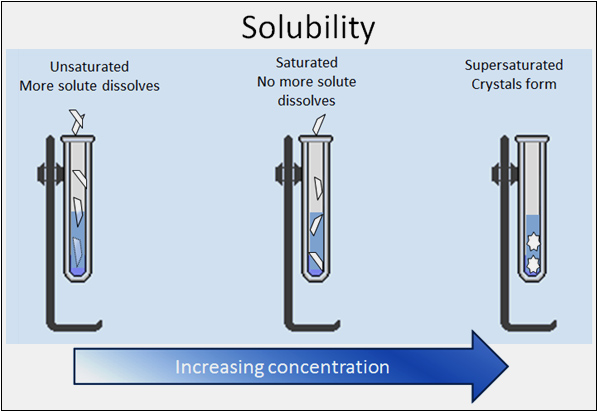
Suppose you pour a glass of cold unsweetened tea. You add sugar and stir. You stop and take a drink. Not sweet enough? If you can add more sugar then your sweet tea is unsaturated. So you add more sugar, and when you turn your head, your little sister adds more. Now you stir and stir and some sugar falls to the bottom. No more sugar will dissolve—your sweet tea is saturated. The only way to add more sugar would be to heat the tea. Hot tea will dissolve more sugar than the same amount of cold tea. If you heated your tea, added more sugar and then let it cool—you would have a supersaturated solution. Some sugar crystals might “fall” out of the solution as it cools.
The graphic below shows a solute dissolving in an unsaturated solution. The second test tube has a saturated solution where no more solute dissolves. The third test tube shows crystals forming in a supersaturated solution.