Source: Precipitation, Rgee’s 6th Grade Blog

Source: Precipitation, Rgee’s 6th Grade Blog
What do you think of when you hear the word precipitation? You most likely have learned that rain, snow, and sleet are all types of precipitation.
In chemistry, the word precipitation has a different meaning: instead of something that falls out of the sky, a precipitate is something that falls out of a solution. One of the ways a recipitate can be formed is when two soluble compounds interact. A soluble compound is one that will dissolve in water. Insoluble compounds do not dissolve in water.
![]() The picture below shows two soluble compounds combining to form an insoluble compound, also known as a precipitate. Use the information in the picture to identify if each compound is soluble or insoluble.
The picture below shows two soluble compounds combining to form an insoluble compound, also known as a precipitate. Use the information in the picture to identify if each compound is soluble or insoluble.
![]() Watch the video below showing another example of a precipitation reaction and how to write the chemical equation for a precipitation reaction. In your notes, answer the following questions as you watch the video.
Watch the video below showing another example of a precipitation reaction and how to write the chemical equation for a precipitation reaction. In your notes, answer the following questions as you watch the video.
Source: Potassium iodide and lead nitrate, Stephen Taylor, YouTube
Interactive popup. Assistance may be required.
The water starts out clear and then the color changes to yellow.
Interactive popup. Assistance may be required.
The water dissolves the compounds, which mean the ions of each compound are separated from each other. The ions are able to recombine and form a new compound, PbI2.
Interactive popup. Assistance may be required.
We can tell that KI is soluble because it readily dissolves in water.
Interactive popup. Assistance may be required.
PbI2 is insoluble. We can tell because it separates out from the water. It does not dissolve.
Interactive popup. Assistance may be required.
The color change indicates that chemical reaction has occurred. In a chemical reaction, the products are different than the reactants, so the ions must have switched places.
The picture below shows the same reaction between KI and Pb(NO3)2.
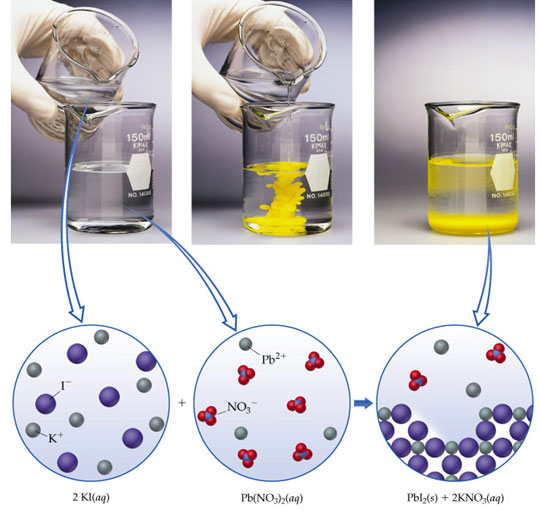
Source: Precipitation Reactions, Prentice Hall
The bright yellow color change makes this a fun chemical reaction to see! Let’s look at the molecular level to understand the chemical reaction better.
Answer the following questions.
Interactive popup. Assistance may be required.
Aq stands for an “aqueous” solution which means a solid ionic compound dissolved in water.
Interactive popup. Assistance may be required.
Each compound is shown split apart into its ions. Different sizes and colors of circles represent the different ions.
Interactive popup. Assistance may be required.
KNO3 is soluble in water. The reaction happens in water, so the K+ and NO3- are dissolved in water and not bonded to each other. If the water evaporated, the ions would bond together.