Source: National Archives and Record Administration
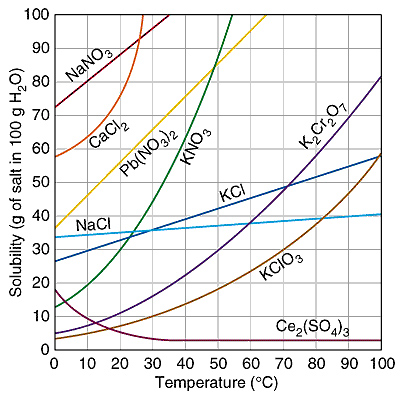
Sample Reading of the Solubility Table
At 20°C, 34 grams of KCl will just saturate 100 grams of H2O. The solubility of KCl at 20°C is 34 grams NaCl per 100 grams H2O.
Use the same skills to read a solubility graph with multiple compounds shown.

Source: National Archives and Record Administration |
Sample Reading of the Solubility Table At 20°C, 34 grams of KCl will just saturate 100 grams of H2O. The solubility of KCl at 20°C is 34 grams NaCl per 100 grams H2O. |