Previously you learned that chemical bonds could either be ionic or covalent. Now let’s look at the two different types of covalent bonds: polar and non-polar. The difference is based on how the electrons are shared between the atoms in the bond. Polar bonds are responsible for many of the unique properties of water, but what causes a polar bond?
![]() Watch the video below and listen for answers to the following questions.
Use your notes to record your answers to these questions.
Watch the video below and listen for answers to the following questions.
Use your notes to record your answers to these questions.
Source: How Polarity makes water behave strangely, TEDEducation
Interactive popup. Assistance may be required.
Water has a very high surface tension because of hydrogen bonding. Water has hydrogen bonding because it is a polar molecule. The hydrogen bonding keeps the molecules close to each other. The weight of insects is not enough to break the hydrogen bonds, but the weight of humans is; therefore, they are not able to walk on water.
Interactive popup. Assistance may be required.
Once again, hydrogen bonding and polarity are the answers! Hydrogen bonding attracts water molecules to each other but keeps them further apart in the solid stage than in the liquid stage. Because the water molecules are further apart as a solid than a liquid, they are less dense as a solid, allowing them to float on water.
Many of the unique characteristics of water are due to its polar nature. Let’s review a little further of what polarity means.
Complete the analogy below that compares the polarity of water to tug-of-war between a toddler and a football player.
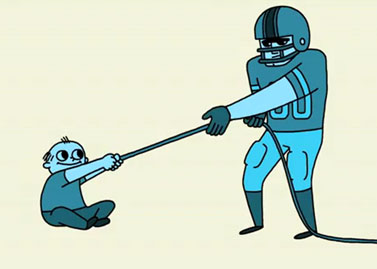
![]() Let’s review what you have learned so far about water and polarity. Complete the following paragraph by selecting the appropriate terms to fill in the blanks.
Let’s review what you have learned so far about water and polarity. Complete the following paragraph by selecting the appropriate terms to fill in the blanks.
The size of the atoms is one reason why the electrons are shared unequally, but electronegativity really helps us understand why oxygen has a greater attraction for electrons than hydrogen.
Water is a covalent compound, not ionic. The two images below both represent the polar bonds in water. Both of the images have strengths and weaknesses. Take a closer look at the drawings to understand how they represent polarity.
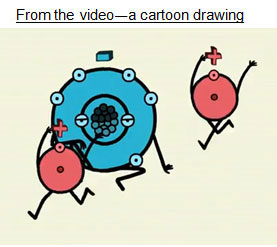
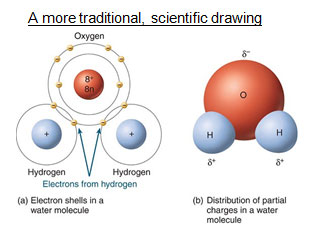
Answer the following questions.
Cartoon Representation
Interactive popup. Assistance may be required.
Oxygen
Interactive popup. Assistance may be required.
Oxygen attracts electrons from hydrogen, so it has more electrons than protons, which results in a partial negative charge on the oxygen.
Interactive popup. Assistance may be required.
Hydrogen
Interactive popup. Assistance may be required.
Hydrogen loses its electrons to oxygen when the elements are bonded, which results in a partial positive charge on hydrogen.
Interactive popup. Assistance may be required.
No, hydrogen is always bonded to the oxygen in a water molecule. This is not an accurate part of the drawing.
Scientific Representation
Interactive popup. Assistance may be required.
δ is a Greek letter that science uses to represent the word "partial."
Interactive popup. Assistance may be required.
The electrons are not fully transferred from hydrogen to oxygen. Water is not an ionic compound, so hydrogen and oxygen are not fully charged. It is most accurate to describe their charges as "partial" charges.