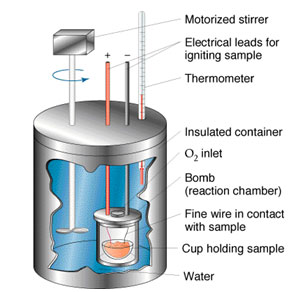
Source: Bomb Calorimeter, University of Florida Chemistry
Calorimetry is the science of measuring the heat of chemical reactions. This is done by use of an instrument called a calorimeter. The image below is an example of a calorimeter.

Source: Bomb Calorimeter, University of Florida Chemistry
You may be more familiar with the type of calorimeter below which is made using two styrofoam cups.
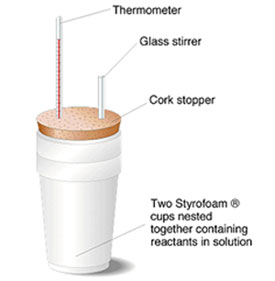
Source: Styrofoam Calorimeter, University of Florida Chemistry
![]() Watch the following two videos to learn how to calculate the heat in a chemical reaction.
Watch the following two videos to learn how to calculate the heat in a chemical reaction.
Part I:
Source: Intro to Chemistry 6.6: Calorimetry (1/2,,Learning4Mastery, YouTube
Part II:
Source: Intro to Chemistry 6.6: Calorimetry (2/2,,Learning4Mastery, YouTube
Now answer the following questions.
If a calorimeter's ΔH is +2001 Joules, how much heat did the substance inside the cup lose? Interactive popup. Assistance may be required.
Since the heat gained by the calorimeter is equal to the heat lost by the system, then the substance inside must have lost the negative of +2001 J, which is -2001 J.
The specific heat of gold is 0.13 J/grams • °C. If 6.00 grams of gold was heated from 20.0°C to 25.0°C, how much heat was applied to the gold? Interactive popup. Assistance may be required.
The formula is
amount of energy = specific heat x mass x change in temperature