Before digging deeper into metallic bonding, let’s review all the bonding types.
![]() Directions: Match the type of bonding to the types of atoms that make up that bond.
Directions: Match the type of bonding to the types of atoms that make up that bond.
One of the common attributes of metals is that metals have only one, two, or three electrons in their outer orbital (valence electrons). When there are only one, two, or three valence electrons, the attraction to the nucleus is rather weak. This weak attraction allows the electrons to "wander" around the atoms of a metal. This sea of delocalized electrons and the attraction to all of the positive ions is the metallic bond, which holds the entire metal structure together.
The animation below demonstrates how the protons form a metallic bond with the sea of delocalized electrons.
How are the positively charged nuclei of the metals not repelled by each other, forcing the atoms to move apart?

![]() Watch the video below for more details on metallic bonds.
Watch the video below for more details on metallic bonds.
Source: Metallic Bonding, trackerchem, YouTube
If you could look at a metal at a molecular level, you would see a swarm of negative electrons randomly buzzing around the positive charged metal ions. This is because of the metal's inability to hold onto its outermost valence electrons.
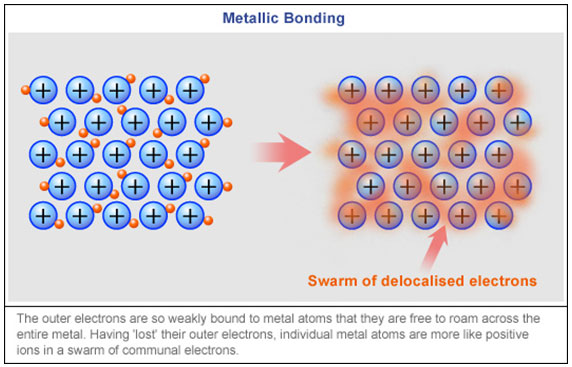
Source: Metallic Bonding, ABC Science
In covalent bonding, there can be a single covalent bond. Is this true for metallic bonding?