Ionic bonding forms through an electrostatic attraction between two oppositely charged atoms, whereas covalent bonding involves sharing of pairs of electrons between atoms. The type of bonding provides clues within their names as to what is happening in the bond. Ionic compounds combine ions. How can you figure out what is happening in covalent bonds? The prefix 'co' means with or together, and 'valent' sounds like valence. This would indicate that the bond has to do with sharing of valence electrons, which is exactly how covalent bonds work.
In 1916, chemist Gilbert Newton Lewis suggested that chemical bonds are formed between atoms because of how electrons, from the bonding atoms, interact with each other. Lewis observed that atoms with fewer than eight electrons in the outer energy level bond together creating eight electrons between them. He noticed that elements are less likely to react when they have eight valence electrons in their outer energy level. Today this type of bonding is called covalent bonding.
![]() Watch the following video to see how oxygen and hydrogen form a covalent bond by sharing electrons.
Watch the following video to see how oxygen and hydrogen form a covalent bond by sharing electrons.
Source: georecitified, covalent_water_hindenberg, YouTube
![]() When hydrogen bonds with oxygen to form water, this is a covalent bond. Since hydrogen only has the first energy level, it only needs two electrons to complete its outer level. Watch the following video to see how water and hydrogen form a covalent bond by sharing electrons.
When hydrogen bonds with oxygen to form water, this is a covalent bond. Since hydrogen only has the first energy level, it only needs two electrons to complete its outer level. Watch the following video to see how water and hydrogen form a covalent bond by sharing electrons.
Source: georecitified, covalent animation, YouTube
Now, let's look at an example with carbon dioxide. The diagram below shows a carbon atom with four valence electrons and an oxygen atom with six valence electrons. Neither has a full outer energy level.
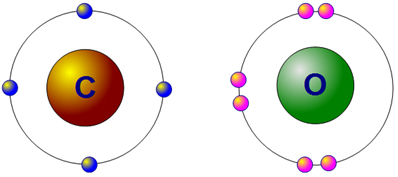
How many electrons does oxygen need to fill its outer energy level?
Interactive popup. Assistance may be required.
2
How many electrons does carbon need to fill its outer energy level?
Interactive popup. Assistance may be required.
4
In the case of carbon dioxide, two oxygen atoms can bond with carbon and share electrons such that all atoms have a full outer energy level, as shown below.
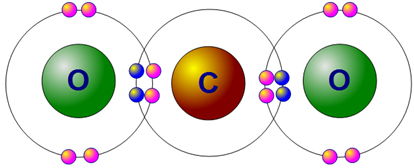
Notice that the oxygen atom to the left shares four electrons with the carbon atom. The oxygen atom on the right does the same. Note that each pair of electrons that is shared is one bond. This compound would be drawn as the following:
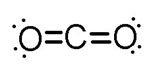
Source: Lewis structure of carbon dioxide, bodrum hotels.com
Covalent bonding most commonly occurs between two nonmetals. Because both nonmetals tend to gain electrons, the atoms involved will share electrons in order to fill the outer energy level.
In terms of electronegativity and ionization energy, how are covalent compounds significantly different than ionic compounds? Look at the two tables showing the electronegativity and ionization energy and look at how nonmetals compare to each other.
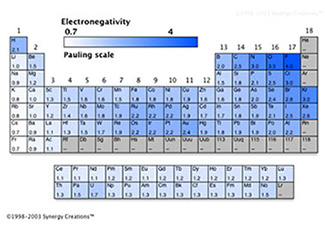 Source: Electronegativity periodic trend, Mr. Everett.com |
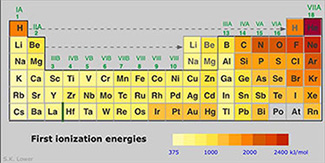 Source: First Ironization Energies, ChemWiki, UC Davis |
Nonmetals have similar values for electronegativity and ionization energy; as a result, elements that form covalent compounds cannot take electrons from each other so are forced to share in order to become more stable with a full valence shell. They can do so by sharing two electrons making a single bond, four electrons to make a double bond, or six electrons to make a triple bond. Remember, each bond is two shared electrons.
Electronegativity continues to play a role in the compound. The most electronegative element in the compound has the greatest strength in holding the electrons that are shared. Think of a game of tug of war when one of the participants is slightly stronger than the other. The rope is being pulled closer to that end. This type of sharing in a covalent compound is referred to as a polar covalent bond. If the elements in a covalent compound are the same there is an equal sharing of electrons. This equal sharing of electrons is referred to as nonpolar covalent compounds.
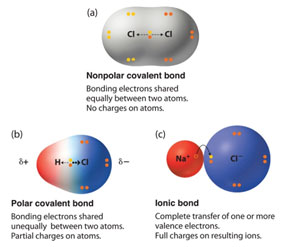
A molecule is the smallest unit of a covalent compound.
Much like ionic compounds, the makeup and structure of a covalent compound leads to particular behaviors. Ionic compounds tend to be solids, but covalent compounds can be solids, liquids, or gases. Covalent compounds lack the crystalline structure that ionic compounds have. This lack in crystalline structure results in covalent compounds having lower melting and boiling points as compared to ionic compounds. Also, they do not dissociate into ions in water; they are not good electrolytes.