![]() Let's take a closer look at potential energy. There are three main types of potential energy: gravitational potential energy, elastic potential energy, and chemical potential energy. Click on the graphic below to learn more about each type of potential energy.
Let's take a closer look at potential energy. There are three main types of potential energy: gravitational potential energy, elastic potential energy, and chemical potential energy. Click on the graphic below to learn more about each type of potential energy.
The more potential energy that is stored in an object, the greater potential there is to do work. Let’s explore the factors that affect the various types of potential energy.
![]() The position or height of an object affects gravitational potential energy. Use the interactive below to explore how height affects gravitational potential energy. The interactive shows a skater on a skate board track. There are three starting positions.
The position or height of an object affects gravitational potential energy. Use the interactive below to explore how height affects gravitational potential energy. The interactive shows a skater on a skate board track. There are three starting positions.
Position |
Height (meters) |
A |
Interactive popup. Assistance may be required.
1

|
B |
Interactive popup. Assistance may be required.
2

|
C |
Interactive popup. Assistance may be required.
3

|
![]() Based on what you learned during this exercise, complete the following conclusion.
Based on what you learned during this exercise, complete the following conclusion.
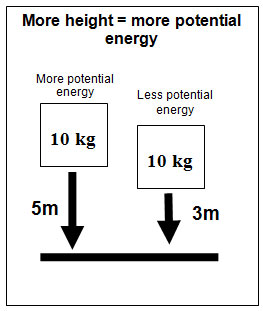
![]() The mass of an object also affects potential energy. Use the interactive below to explore how mass affects gravitational potential energy. The interactive shows three balls with three different masses being dropped from the same height onto foam mat. Click on the "Drop" button to determine which one has more potential energy.
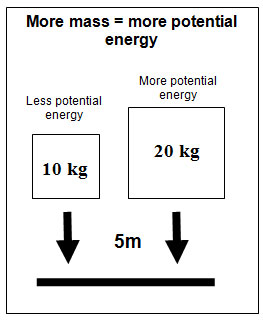
The mass of an object also affects potential energy. Use the interactive below to explore how mass affects gravitational potential energy. The interactive shows three balls with three different masses being dropped from the same height onto foam mat. Click on the "Drop" button to determine which one has more potential energy.
Based on what you learned during this exercise complete the following conclusion.
Which ball had the greatest impact on the foam mat?
Interactive popup. Assistance may be required. the 30 kg ball
![]()
Elastic potential energy can be stored in rubber bands, bungee cords, trampolines, and springs. The amount of stiffness the rubber band or spring has affects its amount of elastic potential energy that is present in the object. The animation below shows springs with 100 gram weights on each.
![]() Click on the release button to see which spring has more potential energy.
Click on the release button to see which spring has more potential energy.
![]() Based on what you learned during this exercise complete the following conclusion statement.
Based on what you learned during this exercise complete the following conclusion statement.
Chemical potential energy is energy stored in chemical bonds. Energy is released when bonds are broken and substances undergo chemical changes. The weaker the chemical bond, the more energy that is present between the bonded atoms.
For example, during respiration glucose sugar is broken down to release energy. During respiration, chemical changes take place, and energy is released.
The formula for respiration is as follows:
The structure of the glucose molecule looks like the image below. Each of the lines represents a chemical bond. If the chemical bonds are broken energy is released.
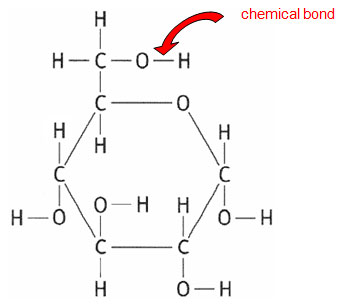
Source: Glucose, BBC