In order to calculate density, you have to know two measurements: the mass and the volume of the object.
To determine the mass of an object, you use a triple beam or electronic balance. Mass is expressed in grams.
If the object is a regular shaped object, such as a cube, you can determine the volume mathematically using the formula for volume. The formula for volume of a rectangular prism or cube is

Volume = B × h (B = area of the base), or Volume = length × width × height
The unit for volume is cm3.
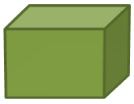
![]() Let’s practice. Determine the volume of the objects below.
Let’s practice. Determine the volume of the objects below.
What if the object is irregular in shape, such as a rock? How do you determine the volume? You cannot determine the volume of an irregular shaped object using a mathematical formula. To determine the volume of an irregular shaped object, we must measure the fluid displacement using a graduated cylinder and water.
![]() Look at the graduated cylinder below. What is the volume of the water?
Look at the graduated cylinder below. What is the volume of the water?
![]() Drag the sample to the graduated cylinder. Record the volume.
Drag the sample to the graduated cylinder. Record the volume.
![]() To determine the volume of the sample, you need to determine how much water was displaced. To do this, you subtract the initial, or beginning, volume from the final volume. How much water was displaced? If you need help click on the word "Hint" below.
To determine the volume of the sample, you need to determine how much water was displaced. To do this, you subtract the initial, or beginning, volume from the final volume. How much water was displaced? If you need help click on the word "Hint" below.
When we use the water displacement method to find the volume of an object, we must convert the units from milliliters (mL) to centimeters cubed (cm3). 1 mL = 1 cm3. So in our example above, 22 mL = 22 cm3.
![]() Now, let’s determine the density of the unknown sample. We know the volume is 22 cm3. Let’s find the mass. Drag the sample onto the electronic balance to determine the mass.
Now, let’s determine the density of the unknown sample. We know the volume is 22 cm3. Let’s find the mass. Drag the sample onto the electronic balance to determine the mass.
Plug the volume and mass into the density formula.

![]()
Since the density formula is mass ÷ volume, the unit for density is grams per cubic centimeter, or g/cm3.
If we have a table of known densities, we can identify the type of material that makes up the sample. Use the table below to determine the material that makes up the sample.
Material |
Density (g/cm3) |
Charcoal |
.2 |
Wood |
.9 |
Brick |
2.4 |
Iron |
7.8 |
Nickel |
8.8 |
Gold |
19.3 |
What was the sample made of?
Interactive popup. Assistance may be required.
brick
In chemistry, the density of substances is often compared to the density of water. In other words, we see if objects float on or sink in water. The density of water is 1.0 g/cm3. If an object has a density greater than 1.0 g/cm3, it will sink. If the object's density is less than 1.0 g/cm3, the object will float.
Based on the density of the sample in the example above, will it float or sink?
Interactive popup. Assistance may be required.
sink