Today's periodic table looks a bit different than Mendeleev's. Scientists know more about the properties of elements than Mendeleev. Also, some of the information found in the periodic table is based on atomic structure which Mendeleev knew nothing about.
The periodic table is a grid of rows called periods and columns called groups. Let's learn a little more about periods and groups.
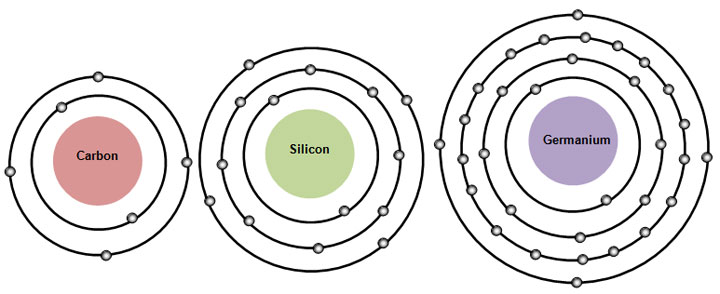
All of the elements in a group have many similar chemical properties. Let's look at drawings of carbon and two other members of its group or family, silicon and germanium. Notice that each of these elements has four valence electrons.

The elements in a group have the same number of valence electrons, or electrons on the outer energy level. This similar electron configuration leads to the elements in the same group having similar chemical properties.