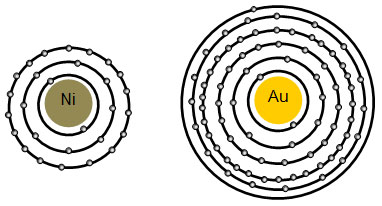
Electrons are located in the electron cloud that surrounds the nucleus. Electrons do not just exist in the cloud in a random way. There is a predictable pattern to the way the electrons exist in the cloud. In order to easily represent atoms, we use diagrams like the ones below. Notice that electrons in the diagram are drawn on circles called energy levels.

Each energy level can only hold a certain number of electrons. Use the following interactive to explore how many electrons the first three energy levels can hold.
Directions: Click on an electron, and drag it to the energy levels. Continue this process until you have placed all the electrons in the atom. Once you have finished, answer the questions below.
How many electrons can the first energy level (one closest to the nucleus) hold?
Interactive popup. Assistance may be required. 2
How many electrons can the second energy level hold?
Interactive popup. Assistance may be required. 8
How many electrons can the third energy level hold?
Interactive popup. Assistance may be required. 18
Can you add electrons to the second energy level without the first being full?
Interactive popup. Assistance may be required. No
Can you add electrons to the third energy level without the first and second being full?
Interactive popup. Assistance may be required. No
As you can see from the interactive, the first energy level can hold two electrons. Eight electrons fill level two, and 18 electrons fill level three. To calculate the number of electrons the other levels can hold, the formula 2n2 can be used. For example, to calculate the maximum number of electrons the fourth level can hold, the equation would be 2(4). The fourth level can hold 32 electrons. In general, each energy level must be full before electrons are placed on the next level. You will learn more about electron configuration when you take high school chemistry.