An atom has three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus of the atom, while electrons are found in the electron cloud. You can determine the number of protons, neutrons, and electrons.
In this section, we are going to explore what makes each element unique and what determines an element's identity.
The following is a simulation from the University of Colorado at Boulder.


Source: Build An Atom, PhET Interactive Simulations, University of Colorado at Boulder
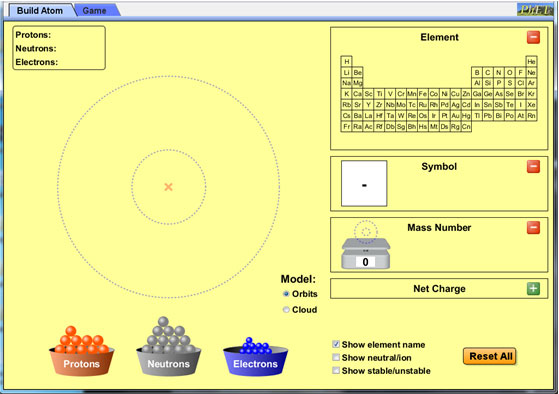
Select the following options on the simulation.
- For the "Model," select "Orbits."
- Click on the green plus icon to expand the following boxes: Element, Symbol, Mass Number.
- Make sure "Show element name" is checked.
Once the correct options have been selected, the simulation should look like the picture below.
- Click on a proton in the proton bucket and drag it to the nucleus.
What is the name of the element with one proton?Interactive popup. Assistance may be required.
Check Your Answer
- Click on an electron and drag it to the orbital of the atom.
What is the name of this element? Interactive popup. Assistance may be required.
Check Your Answer
- Click and drag another proton to the nucleus of the atom.
What is the name of the element?
Interactive popup. Assistance may be required.
Check Your Answer
- Click and drag a neutron to the nucleus of the atom.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
- Click on another electron and drag it to the orbital of the atom.
What is the name of the element? Interactive popup. Assistance may be required.
Check Your Answer
- Click on another electron and drag it to the orbital of the atom.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
-
Click and drag another neutron to the nucleus of the atom.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
- Click and drag another proton to the nucleus of the atom.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
- Click and drag two more neutrons to the nucleus of the atom.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
- Click and drag two more electrons to the orbital.
What is the name of the element?Interactive popup. Assistance may be required.
Check Your Answer
- Continue adding protons, neutrons, and electrons to the atom. Pay close attention to when the element name switches. When you feel like you know what causes the name to switch, answer the question below.


![]()










