As you learned in section one, both elements and compounds are pure substances. This means they are both made of one type of material and have one set of physical and chemical properties.
In this section, we are going to learn more about elements and compounds and their differences.
![]() Watch this video for a brief introduction to elements.
Watch this video for a brief introduction to elements.
Source: Atoms elements and compounds, Drollinger, YouTube
An element is the simplest substance. A list of the elements can be found on the periodic table of elements. Elements contain only one kind of atom. Gold is an element. If you had a bar of gold and you kept breaking it down into smaller and smaller pieces, it would still be gold. Gold is made of nothing but gold.
All elements are represented by a chemical symbol. The chemical symbol is one, two, or three letters. The same chemical symbols are used by scientists all over the world. The first letter of the chemical symbol is always capitalized. Other letters in the symbol are written as lower case. For example, the symbol for hydrogen is H, and the symbol for Sodium is Na.
1
H
Hydrogen
1.008
11
Na
Sodium
22.990
Every element has a unique set of properties that can be used to identify the element. These characteristics are the same no matter what amount of the element is present. These properties include boiling point, melting point, density, and reactivity.
Look at the elements in the table below. You are probably familiar with these elements. These three elements have some similar properties, but each element can be identified by its unique set of properties.
Copper |
Silver |
Gold |
 |
 |
 |
|
|
|
A compound is a pure substance made up of two or more elements that are chemically combined. Elements combine by reacting, or undergoing a chemical change, with one another.
Compounds are represented by chemical formulas. Chemical formulas are a combination of the element symbols that make up that compound. For example, table salt is a compound made of the elements sodium and chloride. The chemical formula for salt is NaCl.
A compound has different properties from the elements that form it. For example, the compound sugar contains the elements oxygen, carbon, and hydrogen. The chart below shows the properties of oxygen, carbon, and hydrogen.
Oxygen |
Carbon |
Hydrogen |
| Colorless Odorless Gas |
Black Solid |
Colorless Odorless Gas |
When these elements combine in the appropriate ratios to form the compound sugar, their properties are not the same at all. The properties of sugar are a sweet, white, crystalline solid. A totally new substance is created even though it contains the elements carbon, hydrogen, and oxygen.

Elements do not randomly join to form compounds. Compounds are formed when elements join together in a specific ratio. The ratio is always the same for the compound. For example, you know that water is made up of 2 hydrogen atoms and 1 oxygen atom. Every molecule of water is made up of exactly 2 hydrogen atoms and 1 oxygen atom. If the ratio were different, it would be a different compound. For example, if there were 2 hydrogen atoms and 2 oxygen atoms, this would be a molecule of hydrogen peroxide. These two compounds only differ by one oxygen atom, yet they have very different properties as seen in the table below.
Water (H2O) |
Hydrogen Peroxide (H2O2) |
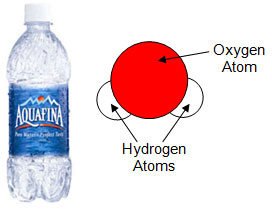 |
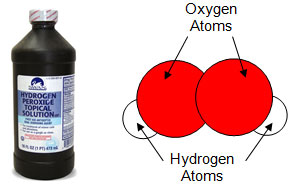 |
Clear Colorless Neutral pH |
Clear Colorless Acid pH |
Compounds can be broken down into simpler compounds and into elements through chemical reactions.
Sources of images used for this section as they appear, top to bottom: