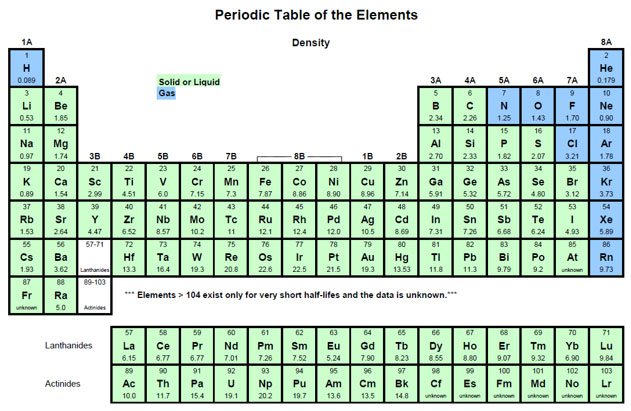
Source: Density Periodic Table, Todd Helmenstine, About Chemistry
Each element has a unique density. Under each of the chemical symbols in the periodic table below, is the density of that element.

Source: Density Periodic Table, Todd Helmenstine, About Chemistry
Since each element has a unique density and density is a size independent property, if you know the density of a substance and are given the volume, the mass can be determined. Likewise, if you know the density of a substance and are given the mass, the volume can be determined.
![]() Let’s look at some example problems.
Let’s look at some example problems.
Now, you try some sample problems.
Element |
Symbol |
Density (g/cm3) |
Mass (g) |
Volume (cm3) |
| Carbon | C |
2.26 |
Interactive button. Assistance may be required.
|
5.62 |
| Nickel | Ni |
8.90 |
Interactive button. Assistance may be required.
|
1.74 |
| Cobalt | Co |
8.86 |
34.61 |
Interactive button. Assistance may be required.
|
| Sulfur | S |
2.07 |
Interactive button. Assistance may be required.
|
3.21 |
| Potassium | K |
0.89 |
47.32 |
Interactive button. Assistance may be required.
|
| Phosphorous | P |
1.82 |
87.32 |
Interactive button. Assistance may be required.
|
| Silicon | Si |
2.33 |
Interactive button. Assistance may be required.
|
271.2 |