Matter is constantly changing. Some changes are physical changes while others are chemical changes.
![]() Watch this video for a brief introduction on the physical and chemical changes in matter.
Watch this video for a brief introduction on the physical and chemical changes in matter.
Source: Matter: Chemical and Physical Changes, Mark Drollinger, YouTube
The chart below summarizes the differences between the physical and chemical changes in matter.
Physical Change |
Chemical Change |
Examples:
|
Examples:
|
During a physical change, only the form or appearance of the matter is altered. For example, the melting of ice is a physical change.
Solid |
Liquid |
Gas |
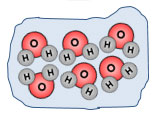 |
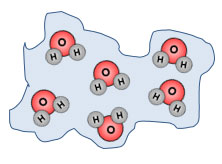 |
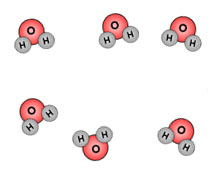 |
Ice is a solid. The molecules of H2O are packed very close together. |
When ice melts, it becomes a liquid. In a liquid, the molecules are not as closely packed as when they are in solid form, but the molecules have not changed in composition. Each molecule is still made up of two atoms of hydrogen and one atom of oxygen. |
When water is heated, evaporation occurs. The molecules spread out even further than they were in liquid form. Water vapor is the gaseous form of H2O. |
In a chemical change or reaction, the original substances' chemical composition is changed. The atoms in the original substances, called the reactants, are rearranged to form completely new substances, which are called the products. The properties of the products are different from the properties of the reactants.

Chemical changes or reactions have been around for centuries. One example of a chemical change that you are probably familiar with is fireworks. The history of fireworks can be traced back thousands of years to China during the Han dynasty. Fireworks have two essential reactants. One reactant is a chemical that is rich in oxygen. Different types of chemicals produce different colors when they are burned. For example, a compound called lithium carbonate, Li2CO3, can be used to produce a red color while a compound called sodium nitrate, NaNO3, can be used to produce a yellow color. The second reactant is a chemical that acts as a source of fuel. Different fuels burn at different rates and temperatures.

Source: Rusting before and after, Corrosionist.com
Iron, Fe, is a metal on the periodic table. Iron has been used since ancient times by humans. The first iron used by humans probably came from meteorites that fell to Earth. The Iron Age, or the time when iron was used as trade, began about 1300-1200 BC. At this time, iron became cheap and available enough to replace bronze for trade purposes. Artifacts, such as iron beads found in Egyptian graves, date back to 5000 BC. Iron rusts every easily, so artifacts made of iron are more rare than artifacts made of silver or gold. Iron rusting is an example of a chemical reaction. During the process of rusting, oxygen reacts with iron. The rust that is produced is a new substance that has different properties than the properties of the iron and oxygen.
The diagram below is a visual model of what happens to the atoms during the process of rusting.
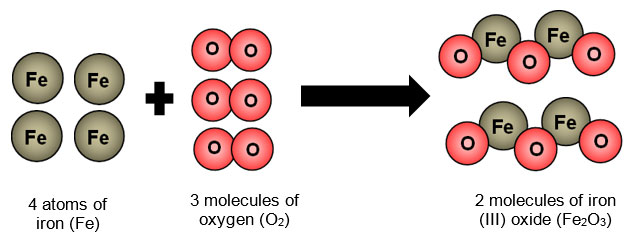
Chemical changes occur when bonds between atoms are broken and/or made. We cannot see the actual breaking or formation of the bonds between the atoms, but we can see other evidence of chemical changes.
Some signs (or evidence) of chemical change are
![]() Watch the following video to see the evidence of chemical changes in a science laboratory.
Watch the following video to see the evidence of chemical changes in a science laboratory.
Source: Indications of Chemical Change, Ed Hitchcock, YouTube
Let's see what you have learned.
![]() Analyze the following descriptions and pictures then, decide if a chemical change has taken place. Describe how you knew it was or was not a chemical change.
Analyze the following descriptions and pictures then, decide if a chemical change has taken place. Describe how you knew it was or was not a chemical change.
Change |
Chemical Change |
Why or Why Not |
When making chocolate milk, the white milk turns brown when the chocolate syrup is added.  |
||
A glass of milk is left out in the sun, and it curdles. The chunky solid forms at the bottom of the glass.  |
||
An antacid tablet is dropped into water, and bubbles are formed.  |
||
 |
||
Water is heated on the stove and the temperature of the water rises from 22°C to 100°C and bubbles are formed in the pan.  |
Change |
Chemical Change |
Why or Why Not |
When making chocolate milk, the white milk turns brown when the chocolate syrup is added.  |
||
A glass of milk is left out in the sun, and it curdles. The chunky solid forms at the bottom of the glass.  |
||
An antacid tablet is dropped into water, and bubbles are formed.  |
||
 |
||
Water is heated on the stove and the temperature of the water rises from 22°C to 100°C and bubbles are formed in the pan.  |

Sources for images used in this section, as they appear, top to bottom: