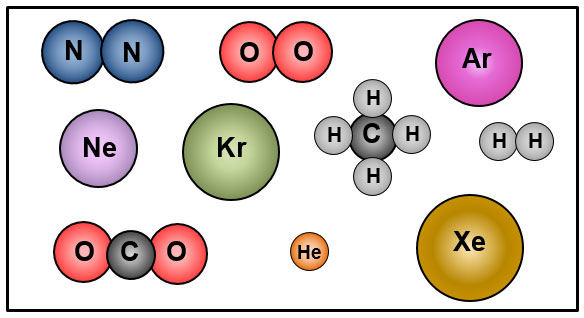
The image below shows models of ten gases that make up the air we breathe.

![]() Which gases are elements and which gases are compounds? Click and drag each gas to the correct location.
Which gases are elements and which gases are compounds? Click and drag each gas to the correct location.
How did you know which gases were elements and which were compounds?
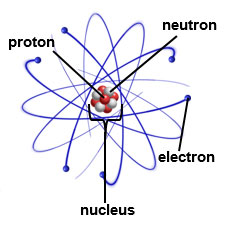
Source: Carbon Atom, Labroots
Atoms are the building blocks of all matter. Atoms are made up of small parts that are called subatomic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons.
The center of the atom is called the nucleus. Protons and neutrons are located in the nucleus. Electrons, which are much smaller than protons and neutrons, spin around the nucleus, leaving lots of empty space in between. Each type of atom has a different number of protons, neutrons, and electrons. For example, the atom Hydrogen (H) has one proton, one neutron and one electron, while the atom Helium (He) has two of each subatomic particle.
![]() Click on the next button and watch the following interactive to learn more about atoms, elements, compounds and mixtures.
Click on the next button and watch the following interactive to learn more about atoms, elements, compounds and mixtures.
![]() Identify the following as elements, compounds or mixtures.
Identify the following as elements, compounds or mixtures.
Sources for images used in this section, as they appear, top to bottom: