Source: Periodic Table of Elements, Stanford University
All matter is made of elements. If there are only 90 natural occurring elements, how are there so many different types of materials?
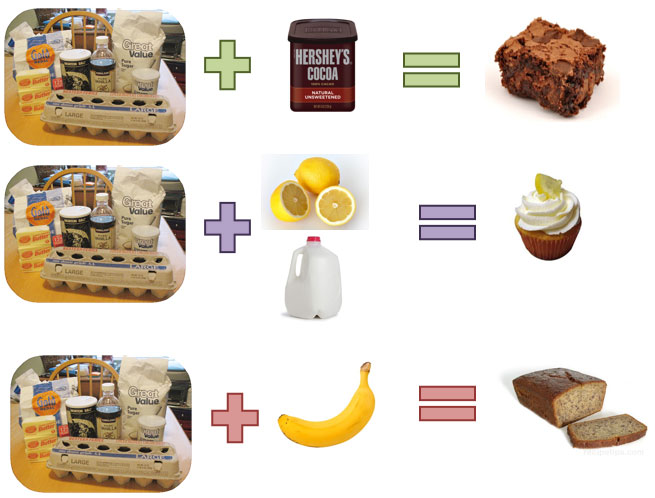
The answer is a lot like baking. Look at the images below.

Source: Periodic Table of Elements, Stanford University
Notice how each of the baked goods starts out with the same main ingredients. Changing the amount of the main ingredients, and adding a few additional ingredients, results in the creation of very different baked item.
The same is true for the composition of the solid Earth, living matter, oceans, and the atmosphere. Each one is made up of a limited number of elements in varying amounts.
The main elements that comprise Earth’s crust, living matter, oceans, and the atmosphere, are highlighted on the periodic table below.
![]() Click on each highlighted square to learn more about that element. Pay close attention to the area of the element fact card that describes where the elements are commonly found.
Click on each highlighted square to learn more about that element. Pay close attention to the area of the element fact card that describes where the elements are commonly found.
Complete the table below, by listing the name and symbol of the elements present in each location. Click on the blanks to check your answers.
| Location | Atmosphere | Living Matter | Crust | Ocean |
| Elements Present | Interactive button. Assistance may be required. | Interactive button. Assistance may be required.
Hydrogen, H |
Interactive button. Assistance may be required.
Oxygen, O |
Interactive button. Assistance may be required. |
Sources for images used in this section as they appear, top to bottom: