
Source: Fire, Awesomoman, Wikimedia Commons
Dirt, Lisa Williams, Flickr
Ancient Greek philosophers such as Plato and Aristotle, believed that everything was made up of four basic parts. Those parts, referred to as the classical elements, are:

Source: Fire, Awesomoman, Wikimedia Commons
Dirt, Lisa Williams, Flickr
During the 4th century BC, the Greek philosopher Democritus came up with the idea that all matter is composed of little indivisible units that he called atoms. Since he thought that the smallest part would always stay together, he named that tiny part "atomos," which means "cannot be cut" in Greek. The atom is the building block of all matter.
Since Democritus' time, scientists have learned that atoms are made up of small parts that are called subatomic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons.
Elements can be found on the Periodic Table of Elements. You have probably seen a periodic table in your science classroom.
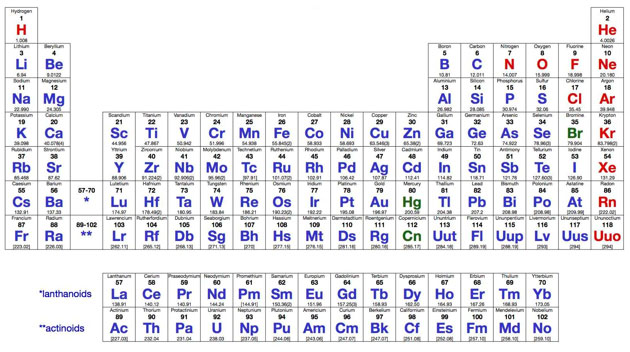
Source: Periodic Table of Elements, Stanford University
One hundred eighteen different kinds of atoms have been identified. Every bit of matter, living or non-living, is made up of one or more of these 118 types of atoms.
Only 90 elements exist outside of chemistry labs. Scientists have made very small amounts of the remaining 28 elements. Scientists create new elements, by bombarding atoms with particles, but these only exist a short time, often only seconds! Since new elements can be created, the periodic table is continually changing.