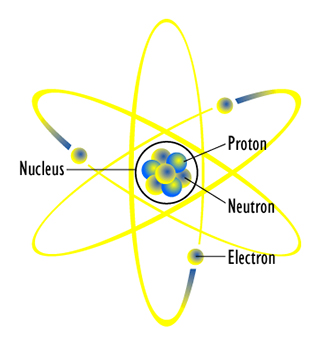
Source: Atom Diagram, astfission, Wikimedia Commons

Source: Atom Diagram, astfission, Wikimedia Commons
One of the most significant steps forward in modern technology is the ability to harness and control the nuclear processes of fission, fusion, and decay. This step forward is deeply propelled by the understanding of Einstein’s mass-energy equivalence.
Fission is the process through which a heavy element like uranium or plutonium is bombarded by high-energy neutrons and forced to split into smaller elements accompanied by a release of energy. Fusion is just the opposite: smaller nuclei like hydrogen are placed under such pressure that they actually fuse together and are also accompanied by a release of energy. Fusion is the process that is occurring in the core of the sun.
Nuclear decay is the process by which unstable nuclei give off mass or energy to try to become stable. Decay is a natural process and occurs all around us.
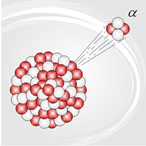
Source: Alphadecay, Burkard HF, |
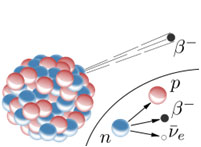
Source: Beca-minus Decay, |
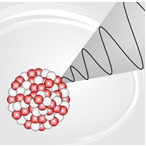
Source: gammadecay-1, Burkhard HF, |
Now that you have had an opportunity to review these nuclear phenomena, let’s take a look at how they are seen in everyday applications.