![]() In the following video, hear Einstein explain his equation.
In the following video, hear Einstein explain his equation.
Source: albert einstein e=mc2 theory of relativity,psychoVid, YouTube
You have heard of the equation that Einstein mentions in the video, E = mc2. This famous equation describes the mass-energy equivalence. In general, mass-energy equivalence states that the mass of a body is proportional to its energy content. It could be said that mass is concentrated energy. This equation is seen in every aspect of our lives, from the radioactive decay necessary for medical scanning to the reactions driving our Sun. It assists us with understanding the relationship between mass and energy.
You will learn about the mass-energy equivalence and the connection it has to nuclear stability, fission, and fusion. Let’s start with a quick chemistry check-in.
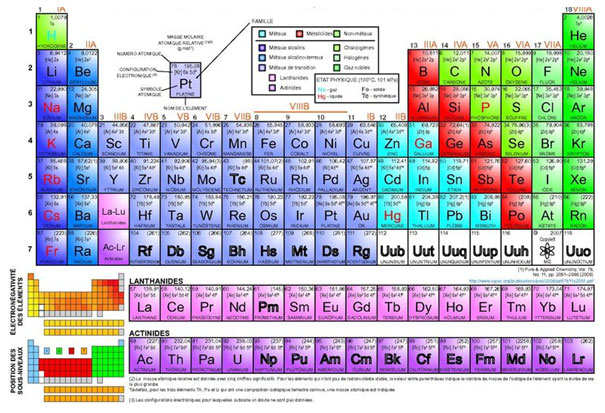
Source: The Periodic Table, Napy1kenobi, Wikimedia Commons
Looking at atomic forces, chemistry and physics are closely intertwined. In order to look more deeply into mass-energy equivalence, let’s take a moment to do a quick chemistry review.
There are 94 naturally occurring chemical elements in nature, from which all of the various compounds and substances on earth are constructed. Each of these elements has unique physical and chemical properties that lead to the huge variety of compounds that can be formed.
In describing these elements, physicists and chemists must specify the following things:
Atomic Number |
Atomic Mass |
The number of protons that are in the nucleus of each atom of the element determines the element. For example, there are always 2 protons in the nucleus of a helium atom, 3 protons in the nucleus of a lithium atom, 92 protons in the nucleus of a uranium atom, etc. |
Protons and neutrons are located in the nucleus of the atom. Protons are all positively charged, and hence, strongly repel each other. The strong nuclear force acts to overcome this force of repulsion, and it is influenced by the number of neutrons in an atom’s nucleus. This means that neutrons, which have no charge, play an important role in the stability of atoms. |
All atoms of an element have the same number of protons, but they can have many different numbers of neutrons. Atoms with the same number of protons but different number of neutrons are called isotopes. All isotopes of uranium have 92 protons in their nucleus—the most common isotopes of uranium have 142, 143, or 146 neutrons in their nucleus.
The image below reviews the basic information found on the Periodic Table of Elements.
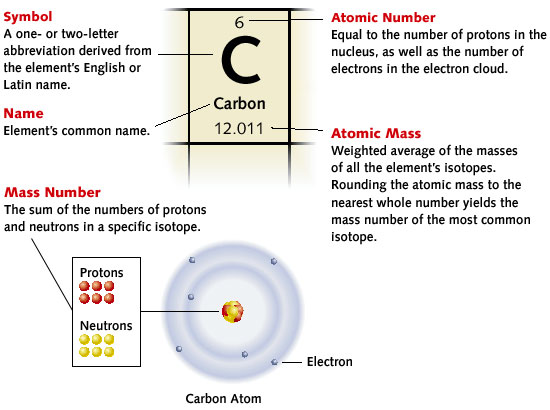
Source: Basic Information found in the periodic table, ClassZone