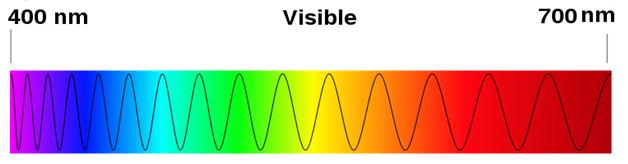
Source: Image adapted from Spectre visible light, SiPlus, Wikimedia Commons
When you look at a white light like a light bulb, through a prism, you will see the continuous spectrum of colors (seen in the top image below), however when you look at the light emitted from a particular element, you will see only a few of those colors or lines highlighted. These colors are part of the emission spectrum for the element (seen in the bottom image below). In this section, we will focus on understanding emission spectra.

Source: Image adapted from Spectre visible light, SiPlus, Wikimedia Commons
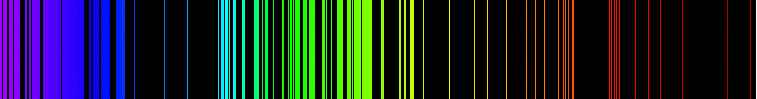
Source: Emission Spectrum-Fe, Artem Karimov, Wikimedia Commons
When an atom is placed in a strong electric field or if it is hit with high energy light, it will sometimes absorb some energy. When energy is absorbed by the electrons of an atom, the electrons will move into a higher energy level, and the atom is said to be in an excited state This excited state is unstable for the atom, so the atom soon returns to the ground state and releases the same amount of energy that was absorbed. This energy is released in the form of a photon visible light. For each type of element, a distinct pattern of visible light is released. This array of visible light is called the emission spectrum for the element. Below is a brief diagram showing a diagram model of this process.

Source: Stimulated Emission, V1adis1av, Wikimedia Commons
![]() Watch the following video where a class is using a specialized tool to observe emission spectra.
Watch the following video where a class is using a specialized tool to observe emission spectra.
Source: Emission Spectrum—2009 BCCP Cosmology Workshop, Artysci101, YouTube
What is the name of the tool used to observe the spectrum?
To see emission spectra of common elements, we use gas discharge tubes similar to the ones seen in the image below and a tool called a spectroscope.
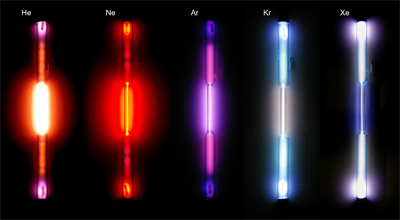
The spectroscope uses a special type of film called diffraction grating to break the light emitted by the element in the gas discharge tube into its components as seen in the visible light spectrum.
