Source: National Archives and Record Administration

Source: National Archives and Record Administration
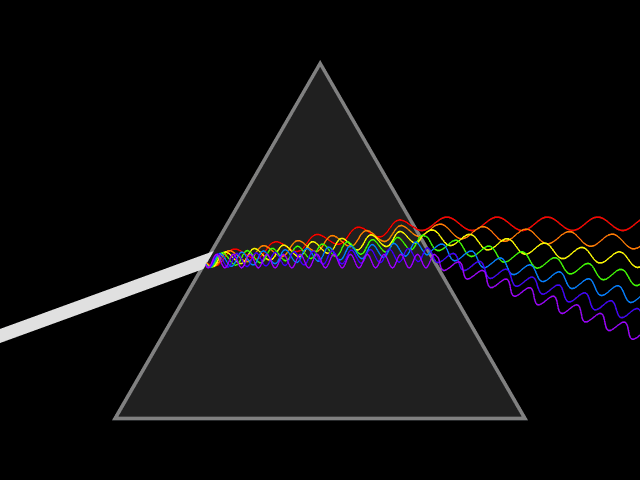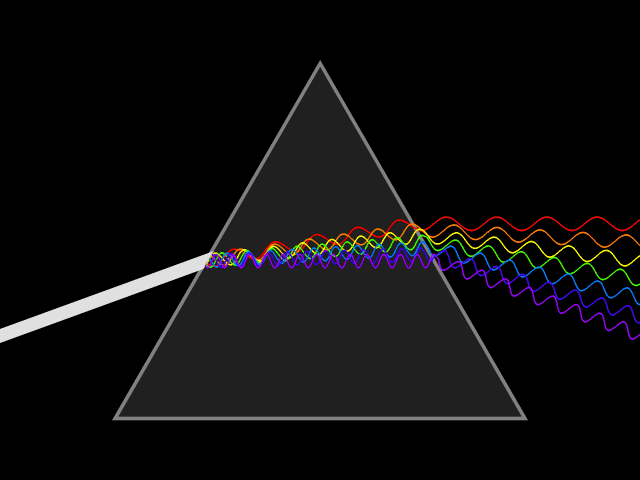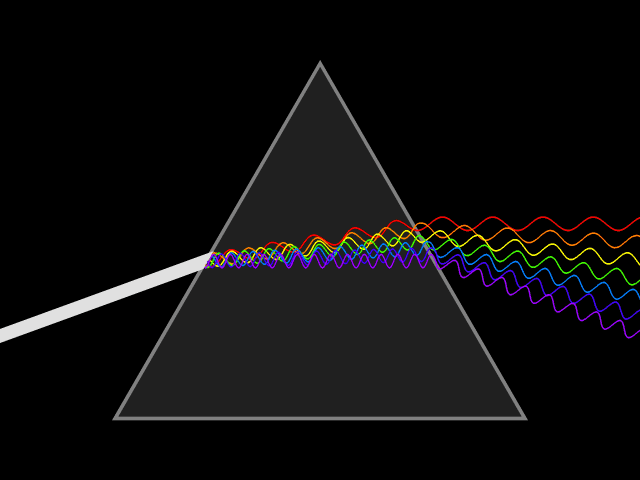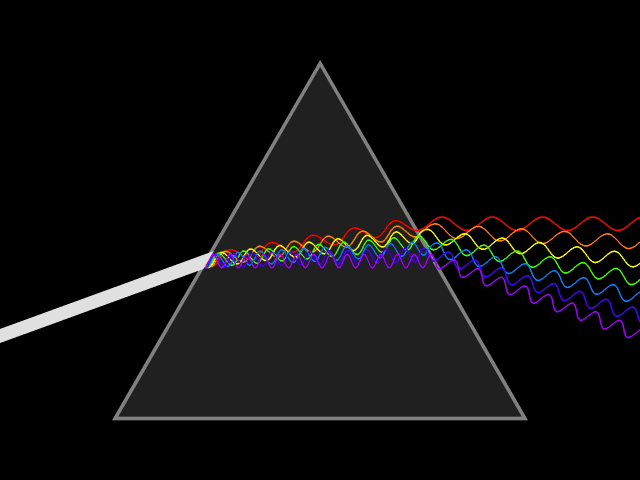
Atomic emission spectra can be observed every day in our lives. When we look at a neon sign, view fireworks in the sky, and when we see images from stars in distant galaxies shared with us by scientists. The animation above shows how white light can be broken down to show the visible light spectrum by using a prism. This visible light spectrum (seen below) is the foundation for the emission spectra produced by different elements. During this lesson, we will explore how emission spectrum are produced, why each element has a "signature" emission spectra (sort of like a fingerprint), and how energy plays a part in the production of the emission spectrum.

Source: Spectrum, Wereon, Wikimedia Commons