 Source: Linear Heat Flow, Tooto, Wikimedia Commons
Source: Linear Heat Flow, Tooto, Wikimedia Commons
Heat is thermal energy transferred from one system or object to another. Heat energy is transferred in three ways: conduction, convection, and radiation. We often confuse heat with temperature. Temperature is the measure of the average kinetic activity of the particles of a substance. If you need more review of the difference between heat and temperature, look in lesson 7 of this module.
Heat transfer is the movement of thermal energy from one object to another. When an object absorbs heat, its particles gain kinetic energy and they vibrate or move faster (remember—kinetic energy is energy of motion.)
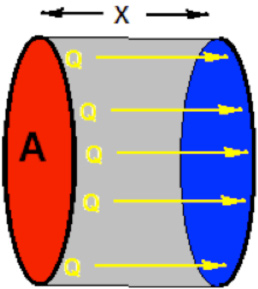
As shown in the following image, heat ALWAYS flows from a warmer area to a cooler area.
 Source: Linear Heat Flow, Tooto, Wikimedia Commons
Source: Linear Heat Flow, Tooto, Wikimedia Commons