 |
 |
| Magnified look at an object with low thermal energy | Magnified look at an object with high thermal energy |
When looking at potential and kinetic energy in molecules, thermal energy and heat should be considered. In this section, you will look at the properties of thermal energy and heat as related to kinetic and potential energy in atoms and molecules.
The thermal energy of a substance is the total of all the kinetic energy of all the atoms and molecules that make up that substance, plus all the potential energy stored in bonds between those atoms and molecules. Whether they are transferring from one part of a gas or liquid to another, or vibrating in their vertex of a crystalline structure, all atoms within matter are always moving.
 |
 |
| Magnified look at an object with low thermal energy | Magnified look at an object with high thermal energy |

![]() Some of this thermal energy is the result of internal reactions, and some comes from the environment. In the following simulation, you increase the thermal energy of two textbooks through friction by rubbing them together:
Some of this thermal energy is the result of internal reactions, and some comes from the environment. In the following simulation, you increase the thermal energy of two textbooks through friction by rubbing them together:
Source: Friction, University of Colorado - Physics
Click the reset button and watch the motion of the individual atoms. What happens as the temperature falls?
Interactive popup. Assistance may be required.
The vibrations of the atoms are slowing down.
Now use your mouse to rub the yellow book against the green book. What happens?
Interactive popup. Assistance may be required.
The atoms begin to vibrate more, and the temperature rises.
As the books are rubbing against one another, some of the molecules seem to "fly off." What do you think is happening?
Interactive popup. Assistance may be required.
The energy is being transferred to heat energy.
Heat is the transfer of thermal energy from one object to another as shown in the image below.
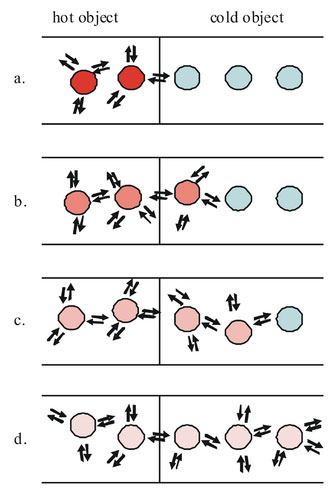
Heat can be measured in Joules, but the more traditional unit is the calorie (lower case c). One food Calorie (upper case C) is 1000 heat calories. Joules and calories are related by the following:
1 calorie = 4.186 Joules
Thermal energy will always transfer from an object with more energy to an object with less energy. Heat travels from object to object, cold does not. When you put ice in a drink, cold does not transfer from the ice to the drink, heat travels from the drink into the ice.
![]() The following video clip provides a demonstration of heat transfer. (You only need to watch the first 186 seconds of the video.)
The following video clip provides a demonstration of heat transfer. (You only need to watch the first 186 seconds of the video.)
As you watch, consider the following:
What is happening on the molecular level?
Interactive popup. Assistance may be required.
The water molecules from the steam vapor being released are transferring energy to the water molecules in the liquid water. This results in the transfer of heat between water molecules.

Source: Heating Water by Direct Steam Injection, HorneSteam, You Tube
Sources of images used for this section as they appear, top to bottom: