Source: Alpha decay, HF Burkhard, Wikimedia
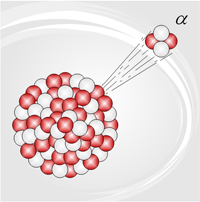
The strong force is the force that holds together protons and neutrons within the nucleus of an atom. Since all protons are positive, they electrically repel one another within the nucleus of an atom. The strong force overcomes this repulsion to keep the nucleus from breaking apart. If the nucleus is too large, it can become unstable and break apart through a process called fission. The strong force is best described by the force carrier model. The protons and neutrons exert this force by exchanging particles called gluons.
The strong force is associated with the energy released during fission and fusion, and the emission of alpha particles during nuclear decay.

Source: Alpha decay, HF Burkhard, Wikimedia
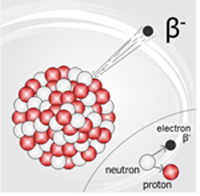
All the stable matter in the universe appears to be made up of one type of lepton (the electron) and two quarks (the up and down), which compose the neutron and the proton. However, six types of each have been predicted and observed. Due to the weak force, scientists have not observed more massive quarks and leptons. It is the weak force that causes massive leptons and quarks to decay—or break down—into lighter leptons and quarks.
The weak nuclear force is mainly observed by scientists under controlled conditions during experiments. The most common example of the weak nuclear force in nature is called beta decay. This occurs when a nucleus becomes unstable because it has the wrong number of neutrons relative to its number of protons. One of the ways that the nucleus can make itself stable is to change a neutron to a proton. This is a complex process, but the final product is a stable nucleus and an extra electron that races out of the nucleus with high energy. This high-energy electron gets a special name—it’s called a beta particle.
Source: modified in Paint from Betadecay, HF Burkhard, Wikimedia
![]() Understanding of the strong nuclear force and the weak nuclear force is relatively new when considering the history of forces. Take a moment to watch a brief introductory video about these fundamental forces.
Understanding of the strong nuclear force and the weak nuclear force is relatively new when considering the history of forces. Take a moment to watch a brief introductory video about these fundamental forces.
Source: The Weak and Strong Nuclear Forces, SciTechUK, YouTube
Now let’s look at a few key scientists that have had a role in understanding nuclear forces. Below is a brief history of the understanding of strong and weak nuclear forces. Click on the links to the scientists and their discoveries to learn more about them. Take notes as you explore the history of strong and weak nuclear forces.
A brief history of the understanding of
strong and weak nuclear forces
|
Ernest Rutherford (1911): Identified atomic nucleus James Chadwick (1932): Discovered the neutron Werner Heisenberg (1932): Created quantum mechanics Enrico Fermi (1934): Proposed weak nuclear force in beta-decay Alexandru Proca (1930's): Added to the development of strong nuclear force with work on equations leading to the development of the theory of atomic nuclei Hideki Yukawa ( (1935): Published his theory on meson hypothesis Peter Ware Higgs (1960's): Proposed the Higgs mechanism Steve Weinberg, Sheldon, Glashow, Abdus Salam (1979): Received the Nobel Prize for work on electroweak theory |