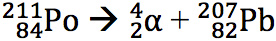 Source: Alpha Decay, Burkhard HF, Wikimedia Commons
Source: Alpha Decay, Burkhard HF, Wikimedia Commons
Alpha:
 Source: Alpha Decay, Burkhard HF, Wikimedia Commons
Source: Alpha Decay, Burkhard HF, Wikimedia Commons
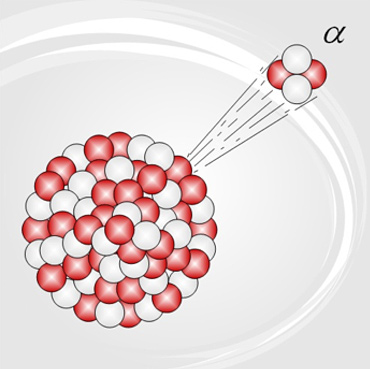
When a nucleus is too large, the electric repulsive force will overcome the strong nuclear force and a small piece of the nucleus–called an alpha particle–will shoot out of the nucleus at very high speed. The alpha particle contains two protons a two neutrons; it has a charge of +2 and a mass of 4 AMU (atomic mass units). It is essentially the same as a helium nucleus, which–as we saw in section 2–is a very stable particle. When an alpha particle is kicked out, the atom's mass is reduced by four, and its atomic number is reduced by 2. This means that it becomes a different element.
Example:
Polonium-211 is a very unstable isotope of polonium that will undergo alpha decay very quickly. The mass is reduced by 4 from 211 to 207, and the atomic number is reduced by 2 from 84 to 82. The atom is therefore changed from polonium to lead.