Source: Alpha Decay, Burkhard HF, Wikimedia Commons

Source: Alpha Decay, Burkhard HF, Wikimedia Commons
alpha (α) |
beta (β) |
gamma (γ) |
| Generally involves a nucleus that is too big | Generally involves a nucleus with the wrong number of protons | Generally involves a nucleus with too much energy |
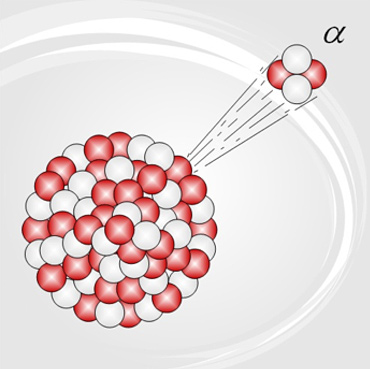
When a nucleus is too large, the electric repulsive force will overcome the strong nuclear force, and a small piece of the nucleus–called an alpha particle–will shoot out of the nucleus at very high speed. For example, in the picture above, the alpha particle contains two protons and two neutrons. It has a charge of +2 and a mass of 4 AMU (atomic mass units). It is essentially the same as a helium nucleus, which is a very stable particle. When an alpha particle is emitted from a nucleus, the atom's mass is reduced by four, and its atomic number is reduced by 2. This means that it becomes a different element.
Polonium-211 is a very unstable isotope that will undergo alpha decay very quickly. The atomic mass is reduced by four from 211 to 207, and the atomic number is reduced by two from 84 to 82. The atom is therefore changed from polonium to lead.
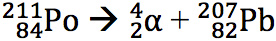

![]() In the following simulation, click on the single atom tab at the top, and watch alpha decay in action.
In the following simulation, click on the single atom tab at the top, and watch alpha decay in action.
What happened to the energy level of the particles in the nucleus after the alpha particle was ejected?
Interactive popup. Assistance may be required.
The energy of the particles was reduced, and the nucleus was stabilized.
alpha (α) |
beta (β) |
gamma (γ) |
| Generally involves a nucleus that is too big | Generally involves a nucleus with the wrong number of protons | Generally involves a nucleus with too much energy |
Source: beta decay, Justin Matis, Lawrence Berkeley National Laboratory
If the nucleus is unstable because of the number of neutrons, the atom will undergo beta decay, which involves the weak nuclear force. Protons and neutrons are made of even smaller particles called up quarks and down quarks. Protons are made with two ups and a down, and neutrons are made with two downs and one up. There are six kinds of quarks total, but only the up and down are stable enough to exist in matter. The weak nuclear force causes one of the down quarks in a neutron to break down into an up quark and a beta particle, which is basically an electron with very high energy. The up quark stays where it is–turning the neutron into a proton, and the beta particle is ejected from the nucleus. Since the neutron turns into a proton, the atomic mass stays the same, and the atomic number increases. As was the case with alpha decay,the atom at the end is a different element.
alpha (α) |
beta (β) |
gamma (γ) |
| Generally involves a nucleus that is too big | Generally involves a nucleus with the wrong number of protons | Generally involves a nucleus with too much energy |
Source: gamma decay, Justin Matis, Lawrence Berkeley National Laboratory
The simplest form of decay is gamma decay. A nucleus that is unstable because it has too much energy–either from recently having completed alpha or beta decay, being struck by another particle or energy, or from some other nuclear phenomena–simply releases some of the energy in the form of gamma radiation. Gamma radiation is a high-energy photon (very high frequency light) that is emitted from the nucleus. The gamma radiation is massless and has no charge. The atomic mass and atomic number of the end product are the same as those of the original atom.