There are four fundamental types of forces: gravitational, electromagnetic, strong nuclear, and weak nuclear. There have been recent developments about forces on an atomic level. These forces are known as the strong nuclear force and the weak nuclear force.
In this section, you will learn more about strong and weak nuclear forces, and in upcoming sections, you will look at the effects of these forces.
![]() The following video takes a look at the four fundamental forces with a specific focus on the two nuclear forces (strong and weak). As you are watching the video, answer the following questions in your notes.
The following video takes a look at the four fundamental forces with a specific focus on the two nuclear forces (strong and weak). As you are watching the video, answer the following questions in your notes.
Source: The Strong and The Weak Nuclear Forces, Maxwell Connor, YouTube
Interactive popup. Assistance may be required.
The strong nuclear force is found in the nucleus of the atom. It is the attraction force that holds neutrons and protons together.
Interactive popup. Assistance may be required.
One way scientists learn about the strong force is through the use of atom smashers, like the Hadron Collider. Scientists use atom smashers to smash atoms together. These atom smashers require a great deal of energy to “blow” the atom apart.
Interactive popup. Assistance may be required.
The weak nuclear force is the attraction force that holds protons and electrons together.
Interactive popup. Assistance may be required.
Scientists use Geiger counters to find electrical charges that are given off when particles leave one atom and “rip” through other atoms.
The strong nuclear force (also referred to as the strong force) is the strongest of what are now understood to be the four fundamental forces of nature. The trade-off, however, is that it also has the shortest range, meaning that particles must be extremely close before its effects are felt.
The strong force is an attraction that holds protons and neutrons together. It is about 100 times stronger than the electric repulsion that the protons exert on each other, but it can only act at distances of a few femtometers (one femtometer is 1*10-15 or 0.000000000000001 meters).
To put this in perspective, the approximate radius on a proton is about 0.85 femtometers. This means that each proton or neutron is only pulling with the strong force on the protons and neutrons immediately surrounding it. Take a look at the image below to see just how small this is.
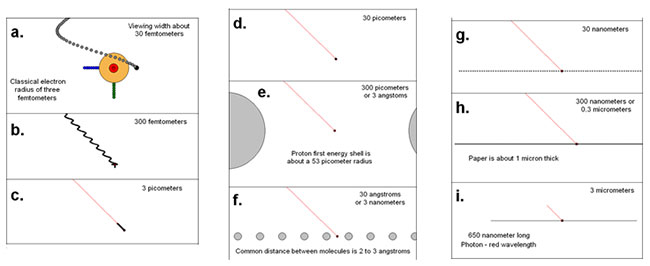
Source: Distance Comparison, Wikimedia Commons
The net result of this short range is that the larger the nucleus, the more likely the electric repulsion is to overcome the strong attraction. This will cause the nucleus to break down. Neutrons tend to help hold a nucleus together because they add to the overall strong attraction but do not add to the electric repulsion. On the other hand, neutrons also make the nucleus larger which–as mentioned above–can make the nucleus more likely to fall apart. In the end, the ideal number of neutrons to help hold a nucleus together is different for each element. This is why you will see stable and unstable isotopes of the same element.
The weak nuclear force (also referred to as the weak interaction) is associated with the stability of a radioactive–or unstable–nucleus. It is responsible for some types of radioactive decay and will be discussed in more detail in section 3.