Source: Atom Diagram, Magnus Manske, Wikimedia Commons
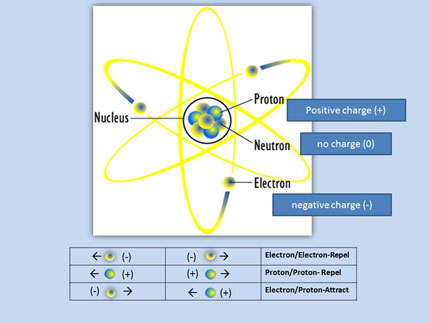
The nucleus of any atom larger than hydrogen is a tightly packed ball of protons and neutrons surrounded by electrons in various energy levels. The electric attraction between the positive protons and the negative electrons keeps the electrons from simply leaving the atom completely. The problem is that like charges repel each other. This means that the protons in the nucleus should push each other away, and the nucleus should fall apart.
This puzzle of "how does a nucleus stay together?" led to the discovery of two nuclear forces.

Source: Atom Diagram, Magnus Manske, Wikimedia Commons