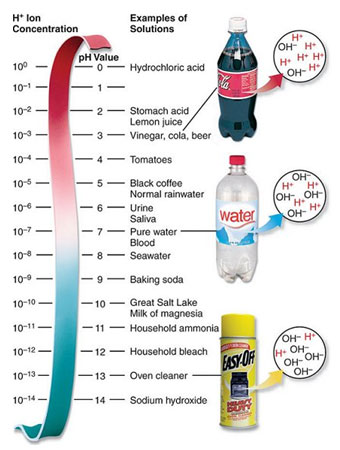
Source: pH Scale, Greg Baxely Chemistry Page

Source: pH Scale, Greg Baxely Chemistry Page
The pH scale is a logarithmic scale. A strong acid can have one hundred trillion (100,000,000,000,000) times more hydrogen ions than a strong base. In order to manage these large numbers, scientists use a logarithmic scale for pH. Because the pH scale is logarithmic, each pH unit below 7 is 10 times more acidic than the next higher value. For example, pH 3 is 10 times more acidic than pH 4, and 10×10, or 100 times more acidic than pH 5. The same holds true for pH values above 7, which are 10 times more alkaline (basic) than the next lower whole value. For example, pH 11 is 10 times more alkaline than pH 10.
Use the pH chart to the right to test your understanding of the pH scale.
Interactive popup. Assistance may be required.
The pH of a solution that is 100 times more alkaline than baking soda is 11; each whole number is ten times more alkaline (10×10).
Interactive popup. Assistance may be required.
The pH of a solution that is 1000 times more acidic than milk of magnesia is 7; each whole number is 10 times more acidic (10×10×10).