
If you’ve ever had a pool in your backyard or worked as a lifeguard, you know that pH is extremely important to the health of the pool. The same is true if you have ever had an upset stomach from too much stomach acid being produced. The tools for measuring pH are known as indicators. Indicators change color depending on the pH.

Phenolphthalein is a common indicator and is colorless. When you add a few drops to a basic solution, it turns pink. If phenolphthalein is added to an acidic or neutral solution, it remains colorless. The image to the left illustrates this.

Bromothymol Blue is another common indicator. As the name indicates, it is a blue colored solution. Bromothymol Blue remains blue in a basic solution. When it is added to a neutral solution, it turns green, and it turns yellow in an acidic solution.
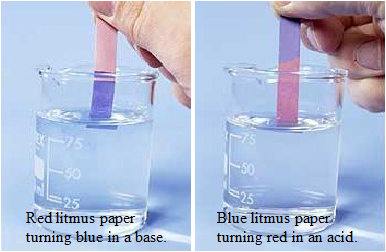
Litmus Paper comes in two colors: red and blue. When dipped into a solution, the color changes, shown in the table below, indicate an acidic or basic solution.
| Red Litmus Paper | Blue Litmus Paper | |
| Acidic Solution | stays red | turns red |
| Neutral Solution | stays red | stays blue |
| Basic Solution | turns blue | stays blue |
 As you can see, most indicators have a limited range of pH. Hydrion paper is very useful in a lab setting where a wider range of pH is needed. Hydrion paper changes color depending on pH. Very acidic solutions are red, neutral solutions are yellow, while very basic solutions are purple.
As you can see, most indicators have a limited range of pH. Hydrion paper is very useful in a lab setting where a wider range of pH is needed. Hydrion paper changes color depending on pH. Very acidic solutions are red, neutral solutions are yellow, while very basic solutions are purple.
Universal indicators use the same scale as Hydrion paper. Watch the video below and describe in your notes the experiment being done.
Why does the solution change color from purple to red?
Interactive popup. Assistance may be required. The solution is initially purple because it contains sodium hydroxide, which is a base. The chemist adds dry ice, which is the solid form of carbon dioxide (CO2). When carbon dioxide is added to water, it forms an acid. This acid begins to neutralize the solution, which causes a green color to be formed. As more and more acid is formed, the solution becomes more and more acidic. More acid is added, finally pushing the solution into the red pH range.
![]() Watch the following video on universal indicators.
Watch the following video on universal indicators.
Source: Universal Indicator with Dry Ice 0809 #1 Base to Acid, Sully Science, YouTube

pH meters are used to make fast, accurate pH measurements.
The picture on the right shows a simple pH meter. A pH meter is a useful instrument. It can be easier to use than liquid or paper indicators. Measurements can normally be made to the nearest hundredth.
Because a pH meter is an electronic device, great care must be used to keep it calibrated and working correctly.
The picture below shows a pH meter making a reading.

Sources used in this section, as they appear, from top to bottom: