![]() Watch the following commercial about the formula in Liquid-Plumr.
Watch the following commercial about the formula in Liquid-Plumr.
Source: Liquid-Plumr: Pipe Snake, 602Communications, YouTube
Is the solution shown in the video an acid or a base? How would you determine the pH of this solution? In this lesson, you will explore the properties of acids and bases and indicators that can be used to determine the pH of a solution.
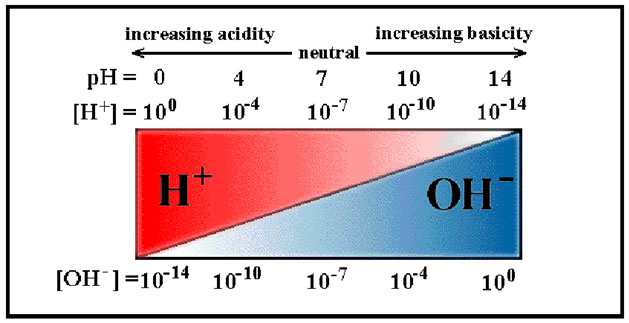
Acids dissociate hydrogen ions (H+) when placed in water. The pH of a solution is a measurement of the concentration of hydrogen ions in a solution. The pH scale measures the acidity or alkalinity of a solution. The pH scale ranges from 0-14. A solution with a pH below 7 is considered an acid. A solution with a pH above 7 is considered a base. A pH of 7 is neutral.
A pH of 7 is considered to be a neutral solution because the concentration of H+ and OH- are equal to one another. As you can see in the picture below, when you move to the left and away from 7, the pH decreases because the concentration of H+ is greater than the concentration of OH-. When you move to the right and away from 7, the pH increases because there is a greater concentration of OH- in solution. Even though a solution may be acidic, there is still a small concentration of hydroxide in the solution. The inverse is true for bases.
Source: pH scale, UTDallas