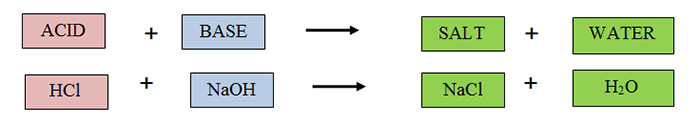
The reaction between baking soda and vinegar is an example of an acid-base reaction. Acid-base reactions are also called neutralization reactions. The products of a neutralization reaction are always a salt (an ionic compound) and water.

![]() Watch the following video to see what it looks like when HCl reacts with NaOH.
Watch the following video to see what it looks like when HCl reacts with NaOH.
Source: Neutralization Reaction, diversitypodcast, YouTube
What is the evidence that a chemical reaction happened when the NaOH was added to the HCl? Interactive popup. Assistance may be required. There was a color change as the two clear liquids turned cloudy. Also, the indicator paper that was used to show the pH of the substances changed.
![]() Practice predicting the products for the following acid-base reactions:
Practice predicting the products for the following acid-base reactions: