The explosive reaction of baking soda and vinegar is an example of what happens when acids and bases mix.
![]() Watch the video below. Watch closely for the formulas of vinegar (acetic acid) and baking soda (sodium bicarbonate) and determine if the formulas fit Arrhenius’ definition of acids and bases. Answer the questions that follow.
Watch the video below. Watch closely for the formulas of vinegar (acetic acid) and baking soda (sodium bicarbonate) and determine if the formulas fit Arrhenius’ definition of acids and bases. Answer the questions that follow.
Source: Chemistry Classics: Acid/Base - Vinegar (Acetic Acid) and Baking Soda (Sodium Bicarbonate), petgraveyard, YouTube


Sodium bicarbonate is just one example of a substance that has many properties of a base, but it doesn’t contain OH- ions. (It turns red litmus paper blue and neutralizes acids.) Therefore, a new definition was needed that would include substances that acted like acids and bases but didn’t necessarily have hydrogen or hydroxide ions in them.

Two scientists, Johannes Bronsted and Thomas Lowry, working separately both developed the same idea: acids and bases could instead be defined as proton donors or acceptors. (Remember that the H+ can also be called a proton.)

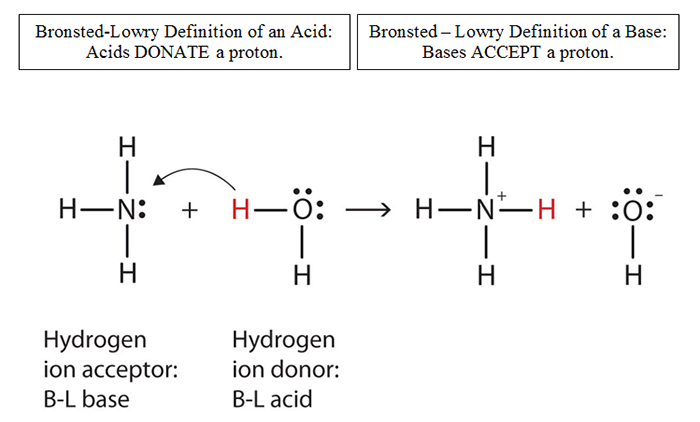
![]() Determine the acid and base in each reaction below.
Determine the acid and base in each reaction below.
Notice that water can act as a Bronsted-Lowry acid or an Bronsted-Lowry base. A substance that can act as an acid or a base is called amphoteric.
Sources of images used for this section as they appear, top to bottom: