
Svante Arrhenius would eventually win a Nobel Prize in Chemistry for his discoveries, but first he had to fight an uphill battle to gather enough experimental evidence to support his ideas. Arrhenius was eventually able to show that when ionic compounds dissolve, they split into ions and this causes the electrolytic behavior of ionic solutions.
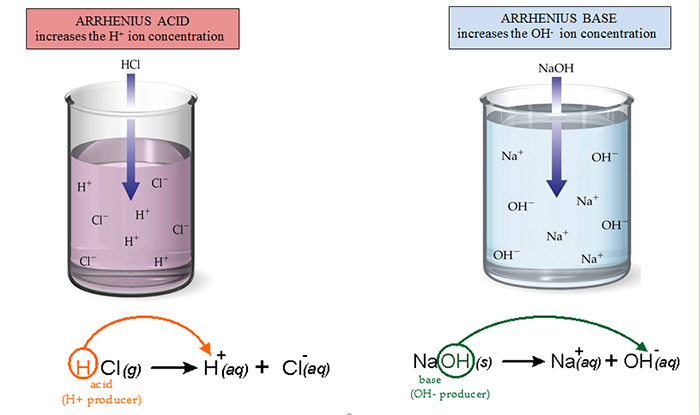
Arrhenius then discovered that acidic substances increased the H+ ion concentration of the solution and basic, or alkaline, substances increased the OH- concentration of the solution. Look at the drawings below showing this transformation.

The pictures above show a model of an Arrhenius acid and an Arrhenius base. Compare and contrast the two pictures and answer the questions below.
The example of the acid produces both H+ and Cl- ions. Which of these ions is responsible for the acidic properties of the solution?Interactive popup. Assistance may be required. H+
The example of the base produces both Na+ and OH- ions. Which of these ions is responsible for the basic properties of the solution? Interactive popup. Assistance may be required. OH-
Explain what causes the difference in properties between acids and bases according to Arrhenius’ definition. Interactive popup. Assistance may be required. The main difference between acids and bases is that the ion concentration increases in the solution. In acids, the hydrogen ion concentration increases in the solution. In bases, the hydroxide ion concentration increases in the solution.
![]() Determine whether the following compounds are acids, bases, or neither.
Determine whether the following compounds are acids, bases, or neither.
An Important note about H+:
Hydrogen ions are highly reactive and do not stay as single ions in water for very long. Hydrogen ions attach to water molecules to form hydronium ions, H3O+ as shown by the reaction below. Hydrogen ions and hydronium ions are often used interchangeably when discussing acids because hydrogen ions form hydronium ions so quickly in solution.
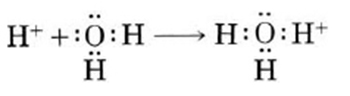
Sources of images used for this section as they appear, top to bottom: