
Source: Coffee, Christy Jordan; Southern Plate

Source: Coffee, Christy Jordan; Southern Plate
In our everyday lives, we describe the strength of solutions using qualitative terms such as “strong” or “weak.” Coffee is one example. Some people like strong, black coffee. Other people prefer to weaken coffee by adding milk or cream.
If you’ve ever made a drink from a powdered mix drink, then you are probably familiar with solution concentrations. If you don’t mix the powder with enough water, the solution will be too strong and will most likely not taste good. If you mix too much water, then the solution will be too weak and flavorless.
Chemists often use solutions of varying concentrations in lab work. The strength of a chemical solution affects how it reacts. Science depends on measurable quantities that can be replicated. Therefore, scientists use molarity as a measure of the strength of a solution. Molarity is the ratio of moles of solute to liters of solution. A strong solution would have a higher molarity than a weak one.
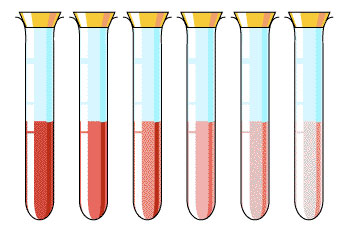
Source: Concentrations, Pascoes Science
Below is the molarity equation as it appears on the Chemistry STAAR Reference Materials.

In order to calculate the molarity of a solution, you will need to know the number of moles of solute and the total volume of the solution.

Source: Scale, JP7Numeracy Blogspot
Source: Beaker with solution, JP7Numeracy Blogspot
Examine the two Material Safety Data Sheets (MSDS) below to see how changing the concentration of the solution changes its reactivity. In your notes, answer the questions that follow.
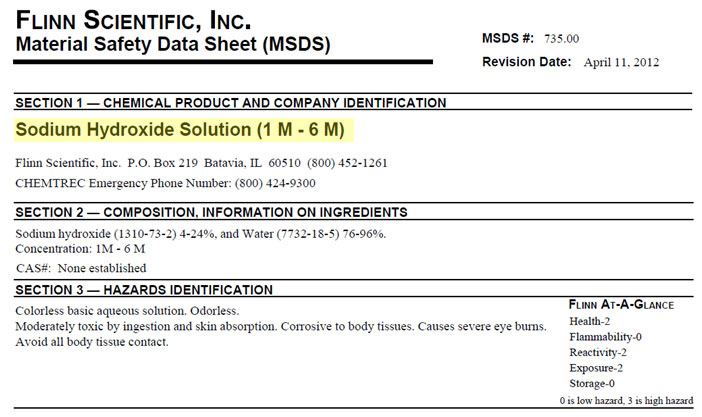

Interactive popup. Assistance may be required.
Sodium Hydroxide or NaOH
6M NaOH 3M NaOH 1M NaOH 0.5M NaOH
Interactive popup. Assistance may be required.
6M NaOH has the greatest moles because it has the highest molarity. 6M means it has 6 moles of NaOH in every 1 L of solution, which is greater than any of the other solutions listed.
In the next section, you will walk through how to use molarity to create a solution and how to solve the sample problems.