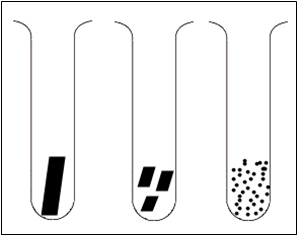
Which of these samples of calcium carbonate would react the fastest with the acid? After you come up with your hypothesis, follow the directions below to perform the experiment to test your hypothesis.
Instructions:
- Click on any of the solids (large lumps, medium lumps or powder) to view the reaction in terms of particle collisions.
- The program can be reset by clicking on the stopper.

![]() Rates of Reaction Surface Area
Rates of Reaction Surface Area
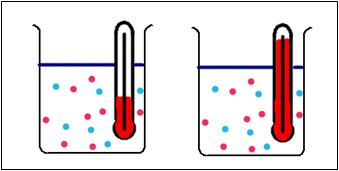
Which of these reactions would occur the fastest, the one at a low temperature or the one at a high temperature? After you come up with your hypothesis, follow the directions below to perform the experiment to test your hypothesis.
Instructions:
- Adjust the temperature of the water bath by rotating the dial. Clicking on the measuring cylinders starts the reaction.
- Reset by clicking the reset button on the water bath.