There are three main factors that control solubility of a solute.
- Temperature - Normally solubility increases with the rise in temperature and decreases with the fall of temperature. The solute will dissolve faster in a solvent at higher temperatures than in the same solvent at lower temperatures.
- Nature of solute or solvent: Remember this: "Like dissolves Like". A polar solute will dissolve in polar solvent. A non-polar solvent will dissolve a non-polar solute.
A non-polar solute will not likely dissolve in a polar solvent. Nor is a polar solvent likely to dissolve in a non-polar solvent.
- Pressure: An increase in pressure increases solubility of a gas in a liquid. For example, carbonated sodas are held in high pressure in bottles or cans. Because of that high pressure, carbon dioxide can be dissolved in the liquid.
Factors affecting the speed or rate in which a solute dissolves.
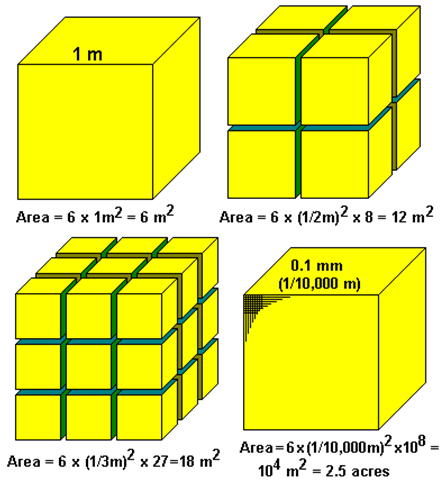
- Surface area, the area of a particle exposed to a liquid or air will affect the speed of dissolving. The image below shows how cutting a large chunk of solute up into smaller pieces, increases the surface area of the solute.
For a one-meter block crushed into 0.1 mm pieces, the edge length is divided by 10,000, and the area multiplied by 10,000. 10,000 square meters is over 100,000 square feet or 2-1/2 acres- Agitation: When we stir a solute in a solvent, it dissolves faster. This is because agitation, stirring, increases the number of collisions between molecules of the solvent and solute.