Many of the substances in your everyday life are solutions. Look at a few examples below. Click on the following pictures to see descriptions.
A solution is a homogenous mixture between two different substances. The two substances are not chemically bonded to each other. There are many types of solutions such as gases dissolved in gases, liquids dissolved in liquids, solids dissolved in solids, and any combination of these. Additionally, solutions can be made of elements, compounds, or both.
![]() Look at the examples of solutions (above) and complete the following chart identifying what type of solution each represents.
Look at the examples of solutions (above) and complete the following chart identifying what type of solution each represents.

Solutions and compounds can be easily confused because they are similar in appearance and both are made of more than one substance. However, they are very different at the molecular level. Look at the pictures to the left that represent elements, compounds, and solutions. Answer the following questions in your notes:
You have made (and drunk) a few solutions too! Think of the last time you made sweet tea or hot chocolate or drank a soda.
![]() In this lesson, you observed what allows a solute (the stuff that gets dissolved) to dissolve faster in the solvent (the stuff that is doing the dissolving). Now, identify the solute and solvent in each of the solutions below. Click in the boxes to check your answers.
In this lesson, you observed what allows a solute (the stuff that gets dissolved) to dissolve faster in the solvent (the stuff that is doing the dissolving). Now, identify the solute and solvent in each of the solutions below. Click in the boxes to check your answers.
Water is the solvent for all these solutions! Solutions that have water as the solvent are called aqueous solutions. These are used often in chemistry.
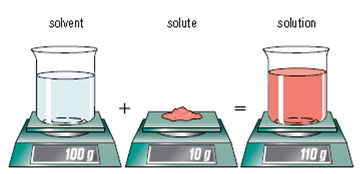
It may appear that the solute disappears, but after closely looking at the mass, as shown in the picture to the left, it reveals that the solute still exists after it is dissolved. It becomes completely surrounded by solvent particles so you can no longer see the difference between the solute and solvent.
What causes one substance to dissolve in another? And what affects the rate at which dissolving happens? Continue to the next section to learn more.
Sources for images used in this section, as they appear, from top to bottom: