 Source: Water molecules, Worsley School
Source: Water molecules, Worsley School
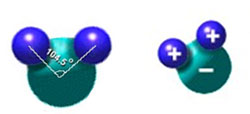
First let's look at why models of the water molecule look a bit like Mickey Mouse's head.
 Source: Water molecules, Worsley School
Source: Water molecules, Worsley School
Water is a polar molecule (a charged molecule with slightly positive and negative ends). The two hydrogen atoms are on one side of the oxygen atom and are separated by an angle of 104.5°. The negative charges are mostly on the oxygen atom, and the hydrogen have a more positive charge. Because of the polarity the water molecule can attract other charged molecules, such as, ionic compounds and other polar molecules.
Watch the following animation to see how water molecules are held together by hydrogen bonds, due to the molecular polarity (charged ends of the molecule).

![]()
The polar nature of the molecules allows liquid water to act in ways other, non-polar liquids do not. The attractive force between molecules makes water molecules more likely to stick to each other rather than something else. This means water has a high surface tension.