

Have you ever stopped to think how interesting it is that ice floats on water? Solids are usually denser than liquid because the atoms are closer together. Therefore, in most substances, the solid form would sink when placed in the liquid form.
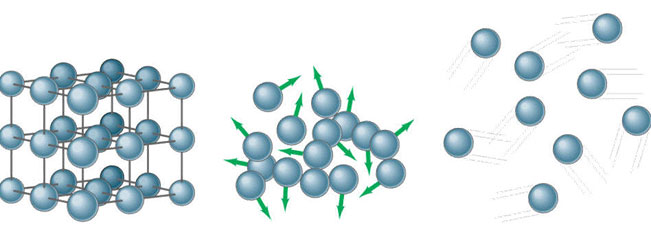
Learn more about why the solid form of water is less dense than the liquid form.
![]() Watch the following video to see how water molecules are held together by hydrogen bonds due to the molecular polarity (charged ends of the molecule).
Watch the following video to see how water molecules are held together by hydrogen bonds due to the molecular polarity (charged ends of the molecule).
Source: Hydrogen Bonding Video, mtchemers, YouTube
![]() Let’s review what you learned from the animation. Answer the questions below.
Let’s review what you learned from the animation. Answer the questions below.
Hydrogen bonding occurs in many compounds, not just water. Hydrogen bonding occurs between any compounds where hydrogen is bonded to N, O, or F on one molecule, and a neighboring molecule has a lone pair of electrons on an N, O, or F.
Below is a cartoon representation of hydrogen bonding.
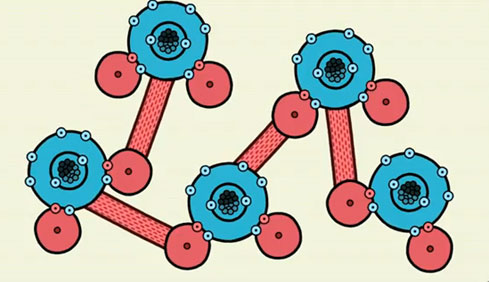
Answer the following question.
Why is the solid state of water less dense than the liquid state of water?
