Source: Test tube, OCAL, Clickr
Now that you are comfortable with the terms "strong" and weak, let’s focus on concentrated and dilute. As mentioned in the previous section, a strong acid dissociates 95 percent to 100 percent of the hydrogen ions. A solution of one gram of sulfuric acid in ten liters of water is a solution of a strong acid. It is also an example of a dilute solution of a strong acid. The word strong refers to the acid's ability to ionize, not the concentration of the solution. By contrast, "concentrated" indicates that there are a very large number of particles—molecules or ions—dissolved in a particular volume of solution.
The concentration of a solution refers to the number of particles per liter of solution. Concentration is measured using the unit molarity.
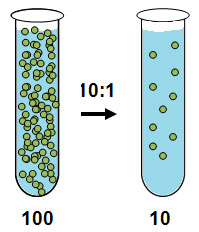
Look at the test tubes below. Each test tube has equal amounts of water. The test tube on the left would be considered a concentrated solution, and the test tube on the right would be considered a dilute solution. Notice when discussing dilute or concentrated there is no mention of dissociation.

Source: Test tube, OCAL, Clickr
Look at the two pictures below. The number of soldiers (particles) within the same size box is what makes one concentrated and the other dilute. The soldiers raising their swords would be representative of particles that have dissociated. As you can see in both pictures, less than 95 percent of the soldiers have raised their swords. In the first box 5 out of 15, or 33.3 percent of the soldiers have raised their swords while in the second picture 1 out of 5 or 20 percent of the soldiers have raised their swords. Remember, a strong acid dissociates 95 percent to 100 percent of the hydrogen ions. Therefore, both solutions pictured here would be considered weak acids.

Source: Concentrated Vs. Dilute Middle School Chemistry