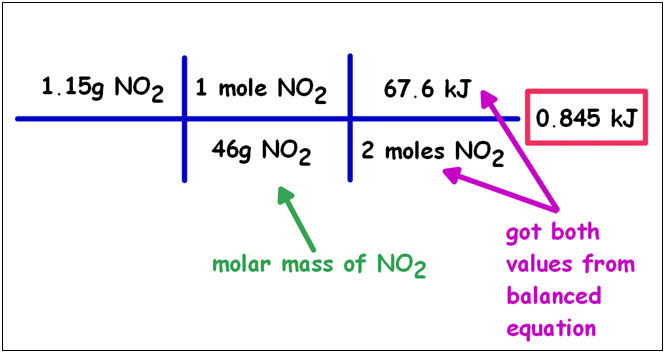
If you have a balanced equation you can convert between mass and energy using dimensional analysis.
Calculate the heat associated with the decomposition of 1.15g of NO2(g) according to the equation:
| N2(g) + 2O2(g) → 2NO2(g) | ΔH = +67.6 kJ |

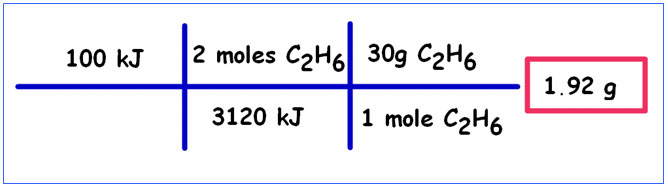
Try this one on your own:
Calculate the mass of ethane, C2H6, which must be burned to produce 100kJ of heat.
| 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) | ΔH = -3120kJ |
Interactive popup. Assistance may be required.
 Close
Close
Is this reaction endothermic or exothermic?
Interactive popup. Assistance may be required. Exothermic, because the ΔH is negative. Close