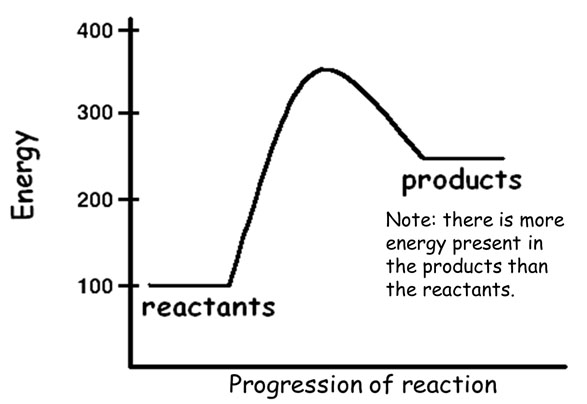
An endothermic reaction is a chemical reaction that absorbs energy from its surroundings.
![]() Watch the following video of an endothermic reaction between barium hydroxide octahydrate crystals with dry ammonium chloride.
Watch the following video of an endothermic reaction between barium hydroxide octahydrate crystals with dry ammonium chloride.
Source: Endothermic reaction, trackerchem, YouTube
The heat absorbed is called enthalpy and is represented by ΔH.
In an endothermic reaction, energy can be represented as a reactant.
X + Y + 150kJ → Z
It can also be represented as having positive enthalpy.
X + Y → Z |
ΔH = 150kJ |
Breaking a chemical bond requires energy; therefore, it is an endothermic reaction.